C. N. Cook1, 2, K. Scheurlen1, J. George1, A. Swearingen1, S. Galandiuk1 1Price Institute of Surgical Research, Division Of Colorectal Surgery, LOUISVILLE, KY, USA 2University Of Louisville, School Of Medicine, Louisville, KY, USA
Introduction:
The incidence of early-onset colorectal cancer (EOCRC), diagnosed before the age of 50, has steadily increased during the last two decades, and approximately 80% of these cancers are sporadic. Obesity is also on the rise within the United States, and a possible link between metabolic dysfunction, inflammation and EOCRC has been suggested.
Within the tumor microenvironment, tumor-associated macrophages (TAMs) are able to regulate inflammation and to produce the anti-inflammatory carcinogenic macrophage-metabolite itaconate. While anti-inflammatory mechanisms and low peroxisome proliferator-activated receptor γ (PPARγ) expression in CRC are associated with advanced tumor stage and progression, the effects of itaconate on cellular metabolism and cancer progression are largely unknown. The aim of this study was to investigate carcinogenic cytokine expression and PPARγ levels in response to treatment with the macrophage-metabolite itaconate in human colon cancer cells.
Methods:
HT29 colon adenocarcinoma cells were plated into 24-well plates and rested for 24 hours. Cells were treated with the itaconate derivatives dimethyl itaconate (DI) or 4-octyl itaconate (OI) over four different incubation periods. Cellular mRNA was harvested, and qRT-PCR was performed, comparing cytokine and transcription factor gene expression in treated cells with untreated control cells resting in cell medium only. Statistical significance was evaluated using a paired t-test and results p<0.05 are displayed.
Results:
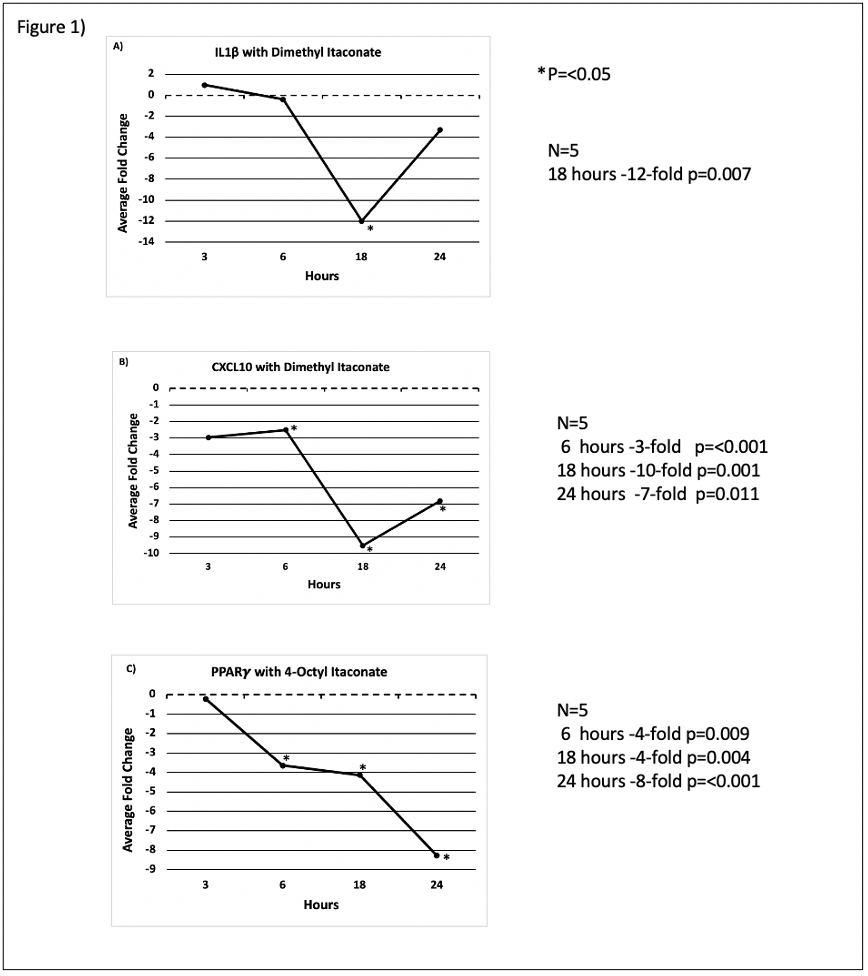
Dl downregulated expression of the proinflammatory cytokine IL-1β at 18 hours (-12-fold p=0.007) (Fig.1A). Proinflammatory CXCL10 was downregulated following DI treatment as well at 6 hours (- 3-fold p=<0.001),18 hours (-10-fold p=0.001) and 24 hours (-7-fold p=0.011) (Fig. 1B), respectively, which can promote tumor development. OI downregulated the transcription factor PPARγ in HT-29 colon cancer cells significantly after 6 hours (- 4-fold p=0.009), 18 hours (-4-fold p=0.004) and 24 hours (-8-fold p=<0.001) (Fig. 1C) of treatment, which is an expression pattern associated with worse outcome in CRC.
Conclusion:
Both itaconate derivatives, DI and OI, induce anti-inflammatory and carcinogenic effects in human colon cancer cells. While DI shifts cytokine expression in colon cancer cells towards a more anti-inflammatory profile, OI directly affects expression of a prognostically relevant transcription factor in CRC. We believe that TAMs mediate cancer-promoting mechanisms through an anti-inflammatory environment and PPARγ downregulation through itaconate in human colon cancer. Macrophage metabolism and itaconate production may link metabolic dysfunction and tumor development in patients with EOCRC.