K. Bittner1, M. C. Mora1, K. Douglas1, K. Wong1, D. Tashjian2, K. Moriarty2, M. Tirabassi2 1Baystate Medical Center,Springfield, MA, USA 2Baystate Children’s Hospital,Springfield, MA, USA
Introduction:
Blood loss is an important factor affecting perioperative outcomes in patients undergoing ex-situ split liver transplantation. The purpose of this study was to determine if monopolar electrocautery can be used to achieve hemostasis in an ex-situ liver transection model.
Methods:
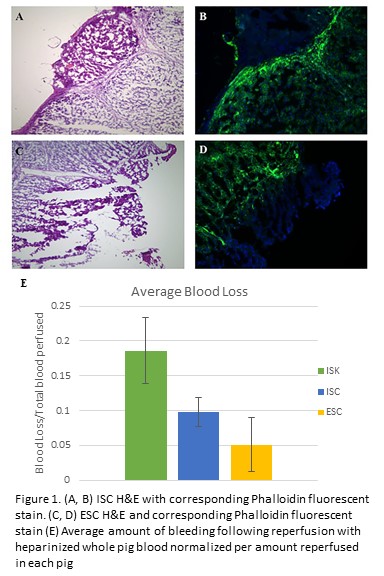
Following euthanasia of Yorkshire swine utilized from an IACUC-approved study, a midline laparotomy was performed. The left hepatic lobe was incised twice to length 6cm and depth 1cm utilizing either monopolar electrocautery (in-situ cautery, ISC) or a 10 blade (in-situ knife, ISK). For ISC, the animal was grounded using a standard adhesive grounding pad. Vascular inflow and outflow to the liver was controlled. The liver was then perfused with heparinized ice-cold lactated ringers. Next, the liver was harvested, transferred to a sterile back table, and placed into a container containing slush solution. The container was set on a draped reusable non-contact grounding electrode pad. Ex-situ, the liver was incised with monopolar electrocautery (ex-situ cautery, ESC) and was then re-perfused with heparinized whole pig blood (203±129mL/pig). After re-perfusion, total blood loss from each liver parenchymal incision was quantified by weight via capture into absorptive gauze. Hematoxylin and eosin (H&E), and fluorescent staining with Phalloidin (F-actin) on liver samples were performed to compare the effects of ISC and ESC.
Results:
In all three re-perfused pig livers, ISK demonstrated the greatest blood loss. In 2/3 pigs, ESC resulted in less bleeding versus ISC. As each pig had a different amount of blood reperfused, data was normalized as a percentage of reperfused blood for each pig (Figure 1E). In ESC and ISC, H&E sections of livers revealed fragmented margins, cell distortion, and elongated nuclei consistent with electrocautery injury (Figure 1A&C). Staining with Phalloidin in both groups revealed a zone of denatured actin corresponding to areas of electrocautery use (Figure 1B&D).
Conclusion:
Monopolar electrocautery can effectively be performed in ex-situ liver transections using a commercially available non-contact grounding electrode pad. Incorporation of this technique in ex-situ liver procedures can potentially result in reduced blood loss after transplantation.
Y. Liu1, V. Cole1, F. W. Sellke1, J. Feng1 1Rhode Island Hospital,Cardiothoracic/Surgery,Providence, RI, USA
Introduction: We have found recently that diabetes is associated with inactivation of endothelial KCa channels, which may contribute to endothelial dysfunction in diabetic patients at baseline. In the current study, we further investigated the effects of cardioplegic ischemia and reperfusion (CP) and CPB (cardiopulmonary bypass) on coronary arteriolar responses to the calcium-activated potassium channel (KCa) opener NS309 in diabetic and non-diabetic patients undergoing coronary artery bypass grafting (CABG).
Methods: The protein expression/localization of KCa channels in the harvested atrial tissue were assayed by Western blotting and immunohistochemistry. Coronary arterioles from the harvested right atrial tissues were dissected pre- and post-CP/CPB from diabetic and non-diabetic patients (n = 5-6/group) undergoing CABG surgery. In-vitro relaxation response of pre-contracted arterioles was examined in the presence of the selective small (SKCa) and intermediate (IKCa) conductance KCa opener NS309 (10-9-10-5 M) and other vasodilatory agents.
Results: There were no significant differences in the total protein levels of IKCa, and SKCa between diabetic and non-diabetic groups or between pre- and post-CP/CPB (P>0.05). The relaxation response to NS309 post-CP/CPB was significantly decreased in diabetic and non-diabetic groups compared to their pre-CP/CPB responses, respectively (P<0.05). Furthermore, this decrease was greater in the diabetic group than that of non-diabetic group (P<0.05).
Conclusion: Our data suggest that diabetes further inactivates KCa channels of coronary microvasculature early after CP/CPB and CABG surgery. This alteration may contribute to post-operative endothelial dysfunction in diabetic patients after cardiac surgery.
A. MacDonald1, K. P. Samy1, B. S. Parker1, T. Truong3, M. Kuchibatla3, J. Espinosa1, M. McRae1, J. Cheeseman1, A. D. Kirk1,2, T. V. Brennan1 1Duke University,Department Of Surgery,Durham, NC, USA 2Emory University,Department Of Surgery,Atlanta, GA, USA 3Duke University Medical Center,Biostatistics And Bioinformatics,Durham, NC, USA
Introduction:
Serum creatinine (sCr) remains the primary screening tool for renal transplant rejection, increasing only after significant damage has been done to the allograft. Acute injury-specific markers may provide an earlier and more sensitive measure of rejection. Urinary N-acetyl-beta-D-glucosaminidase (NAG) is produced in the nephron proximal tubules, which are injured during renal allograft rejection. We retrospectively analyzed urine samples from renal transplant patients to investigate its use as a biomarker for allograft rejection.
Methods:
We retrospectively analyzed 126 urine samples from renal transplant patients collected at two institutions for urinary NAG activity and Cr levels. Raw urinary NAG activity and NAG activity corrected for urinary Cr were correlated to clinical outcome. Analyses were run both with the raw NAG values and also corrected for urine concentration by dividing by the urine creatinine (corrected-NAG).
Results:
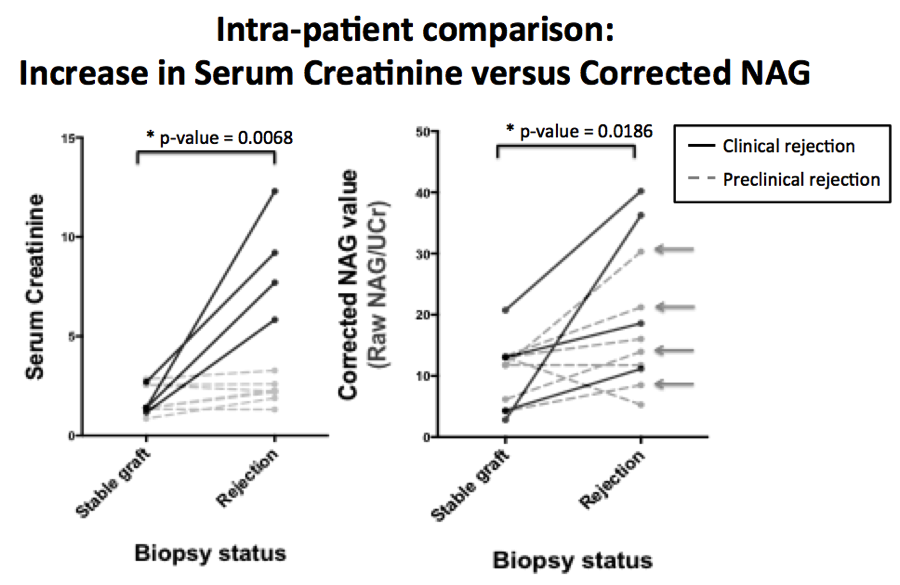
There was a statistically significant elevation in urinary NAG between healthy controls and transplant patients when samples were tested independently (raw NAG p=0.028, corrected-NAG p=0.003). There was a statistically significant increase from a patient’s baseline (during normal graft function) to periods of rejection within single patients for both raw NAG (p=0.002) and corrected-NAG (p=0.02) (Figure 1). Many patients with confirmed histological rejection on protocol biopsy had significant elevations in corrected NAG activity without elevation in sCr (Arrows, Figure 1). There was no significant difference between stable allograft and rejection NAG activity when testing independent samples (raw NAG p=0.143, corrected-NAG p=0.488). Elevations in NAG also occurred in the setting of BK nephropathy.
Conclusion:
Urinary NAG activity was associated with renal injury in transplant patients, with an acute elevation in NAG activity observed in the setting of biopsies showing rejection or BK nephropathy. However, it was not specific for acute rejection versus acute kidney injury from sources such as BK virus. Importantly, urinary NAG was elevated in patients without changes in sCr, indicating that it may serve as an earlier and more sensitive marker of kidney injury than sCr in the workup of a transplant patient.
M. M. Tabbara1, L. Martinez1, J. C. Duque2, A. Paez1, G. Selman1, O. C. Velazquez1, L. H. Salman3, R. I. Vazquez-Padron1 1University Of Miami,Surgery,Miami, FL, USA 2University Of Miami,Medicine,Miami, FL, USA 3University Of Miami,Interventional Nephrology,Miami, FL, USA
Introduction: Intimal hyperplasia (IH) has been historically associated with improper venous remodeling and stenosis after arteriovenous fistula (AVF) creation. However, we recently demonstrated that IH does not explain brachiobasilic AVF maturation failure. Herein, we seek to evaluate whether IH plays a role in AVF focal stenosis. This study compares IH lesions in stenotic and nearby non-stenotic segments collected from the same AVF.
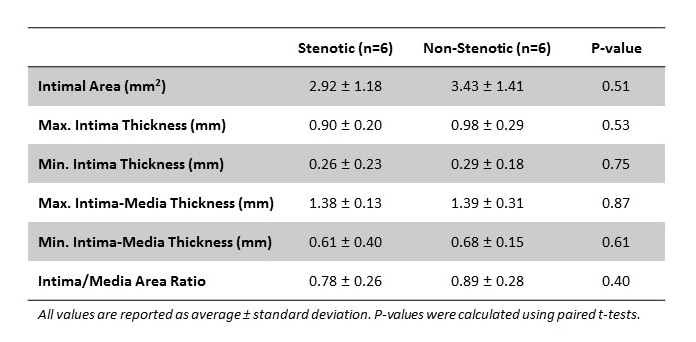
Methods: Focal areas of stenosis were detected in the operating room in patients (n=6) undergoing the second stage basilic vein transposition procedure. The entire vein was inspected and areas of stenosis were visually located with the aid of manual palpation and hemodynamic changes in the vein peripheral and central to the narrowing. Stenotic and non-stenotic segments were documented by photography before tissue collection (6 tissue pairs). Intimal area and thickness, intima-media thickness (IMT), and intima to media area ratio (I/M) were measured in hematoxylin and eosin stained cross-sections, followed by pairwise statistical comparisons.
Results: The intimal area in stenotic and non-stenotic segments ranged from 1.25 to 4.82 mm2 and 1.67 to 5.30 mm2, respectively. There was no significant difference between these two groups (p=0.51). The maximal intima thickness (p=0.53), maximal IMT (p=0.87), and I/M (p=0.40) were also similar between both types of segments.
Conclusion: Despite the present limitations with the small number of samples, these findings concur with our previous study and suggest that IH is not associated with the development of focal venous stenosis in two-stage brachiobasilic fistulas. Our group is actively recruiting additional patients to extend these preliminary analyses.
M. P. Fischbein1 1Stanford University,Cardiothoracic Surgery,Palo Alto, CA, USA
Introduction: Systematically dissect the prenylation pathway to better define the mechanism behind the beneficial effect of statins on aneurysm reduction in MFS and anticipate this will help elucidate the pathophysiology of aneurysm formation.
Methods: Fbn1C1039G/+ mice (4 week old) were treated subcutaneously with either (a) Pravastatin (PS) (HMG-Co Reductase inhibitor) (100 mg/kg per day); (b) Manumycin A (MA) (FPT inhibitor) (2.5 mg/kg/every other day); (c) Perillyl Alcohol (PA) (GGPT-1 and -2 inhibitor) (5.0 mg/kg/every other day); or (d) vehicle control Fbn1C1039G/+ mice from age 4-8 weeks. Aortic dimensions were measured with transthoracic echo.
Results: PS and MA significantly reduced aneurysm growth compared to vehicle control (PS:1.57 ± 0.03 mm; MA: 1.55 ± 0.06 mm; vehicle: 1.77 ± 0.05 mm, respectively: p < 0.05). There was no significant difference between PS and MA treated groups. In contrast, PA did not significantly decrease aneurysm size (PA: 1.81 ± 0.06 mm). Elastin staining illustrated reduced elastin breakdown in MA treated mice compared to vehicle control treated groups (MA: 2.2 ± 0.3, vehicle: 4.2 ± 0.6, respectively: p < 0.05). After identifying that the Ras pathway is important, we measured the relative expression of pRaf-1 and pErk1/2, downstream enzymes in transforming growth factor- β (TGF-β) signaling pathway with WES. Although elevated in control Marfan mice, both pRaf-1 and pErk 1/2 were significantly reduced in MA treated mice, corresponding with a reduction in aneurysm growth (pRaf-1: MA: 5.1 ± 1.3, vehicle: 8.8 ± 0.8, p = 0.08, pErk1/2: MA: 2.3 ± 0.1, vehicle: 3.2 ± 0.1, p < 0.05, respectively
Conclusion: Statins reduce aortic aneurysm growth in Fbn1C1039G/+ Marfan mice by decreasing both Ras activation and downstream ERK signaling
Z. Liu1, P. P. Parikh1, H. Shao1, Y. Li1, R. Prokupets1, S. Liu1, O. C. Velazquez1 1University Of Miami,Surgery,Miami, FL, USA
Introduction: Wounds heal in coordination of a myriad of types of cells, including keratinocytes, inflammatory cells, endothelial cells and fibroblasts. Fibroblasts play pivotal roles in supporting wound healing and tissue remodeling by producing extracellular matrix proteins (ECM) and regulatory soluble factors, providing structural scaffolding and contracting the wound. However, the molecular mechanisms that determine the function of fibroblasts in wound healing remain unknown. Here, we uncover the Notch1 signaling pathway as a molecular determinant that controls the function of fibroblasts in wound healing and angiogenesis.
Methods: To explore the role of Notch1 signaling in regulating function of fibroblasts in wound healing and neovascularization, we generated two new mouse lines in which Notch1 signaling is specifically activated (Gain-Of-Function, GOF) or inactivated (Loss-Of-Function, LOF) in fibroblasts, respectively. A 6-mm skin wound was created on mouse dorsal skin by punch biopsy (n=6/group). Wound healing rates were evaluated based on daily photography. Wound tissue angiogenesis was assessed by Dil-LDL perfusion and confocal microscopy.
Results: Using novel mouse models, we demonstrated that Notch1 pathway activity could determine the function of fibroblasts in wound healing and angiogenesis. Notch1 activation in fibroblasts significantly inhibited wound angiogenesis (~40%, P<0.05) and wound closure rate (~30%, P<0.05). However, inactivation of Notch1 signaling in fibroblasts did not promote angiogenesis and wound healing, likely because the basal level of Notch1 activity in fibroblasts is already very low, lower Notch1 activity by deletion of Notch1 would not be expected to add additional effect.
Conclusion: These findings identify the Notch1 signaling pathway as a molecular determinant that regulates the function of fibroblasts in wound healing and angiogenesis, unveiling Notch1 pathway as a potential target for therapeutic intervention in delayed wound healing and/or vascular diseases.
A. Flores Huidobro1, C. Ibarra1, A. S. Munoz-Abraham1, A. Bertacco1, R. Patron-Lozano1, A. Alkukhun1, R. Morotti1, J. Zinter1, F. D’Amico1, D. Mulligan1, J. Geibel1, M. I. Rodriguez-Davalos1 1Yale University School Of Medicine,Surgery,New Haven, CT, USA
Introduction: Intestinal function may be compromised for several reasons leading to a high number of adult and pediatric patients with intestinal failure. In an effort to reduce injury we have developed multiple animal and human models for extracorporeal perfusion using our intestinal perfusion unit (IPU). We previously published a feasibility study, here we present a comparison between transportation modalities: cold ischemia times, and a variety of preservation solutions.
Methods: Twelve human intestines were procured from our two regional organ procurement organizations using our approved IRB protocol. Eight intestines were procured and connected to the IPU on site. Four intestines were procured, packed in static cold preservation and delivered to our institution within an average time of 8 hours and 42 minutes and the intestines were all connected proximally to jejunum and distally to the ileum, as well as to the superior mesenteric artery in a dual pump system (luminal and vascular). The human intestines were connected to the IPU and perfused with UW (University of Wisconsin) solution, HTK (Histidine-tryptophan-ketoglutarate) and a combination of UW + Ringer Lactate. Samples were taken at 8, 10 and 12 hours in hypothermic perfused conditions. Pathological analysis was determined using the Park/Chiu (P/C) scoring system for intestinal injury (0=normal, 8=transmural infarction).
Results:Histological analysis of intestines shipped and then connected to the IPU showed a P/C score of jejunum (2.3) and for ileum (2.8). Intestines connected to the IPU on-site: P/C score of jejunum (1.85) and for ileum (1.23).. Average cold ischemia time (CIT) for recovered intestines by our team was 2.16 hours and CIT for intestines by other teams was 8.42 hours. Of the 12 total intestines, six were perfused with UW solution, five with HTK and one with UW + RL. UW perfusion had an average P/C score of 1.6 and 1.77 in ileum and jejunum respectively. HTK had a P/C score average of 1.78 in ileum and 2.27 in jejunum. The combination of UW and RL had the poorest score, 2.33 in both the ileum and jejunum.
Conclusion:Continuous hypothermic perfusion of intestinal tissue with UW solution proved to be the best source for limiting ischemia reperfusion injury. Lower ischemia injury scores were seen in the Ileum in comparison to Jejunum. This study demonstrates the advances of the IPU project and the variables that can significantly impact the preservation of the intestinal tissue. The results show that if possible, the intestine should be connected at the procuring site in order to achieve maximal preservation.
D. D. Aufhauser Jr1, D. Murken1, Z. Wang1, G. Ge1, T. Bhatti3, W. Hancock2,3, M. Levine1,4 1University Of Pennsylvania,Surgery,Philadelphia, PA, USA 2University Of Pennsylvania,Pathology And Laboratory Medicine,Philadelphia, PA, USA 3Children’s Hospital Of Philadelphia,Pathology And Laboratory Medicine,Philadelphia, PA, USA 4Children’s Hospital Of Philadelphia,Surgery,Philadelphia, PA, USA
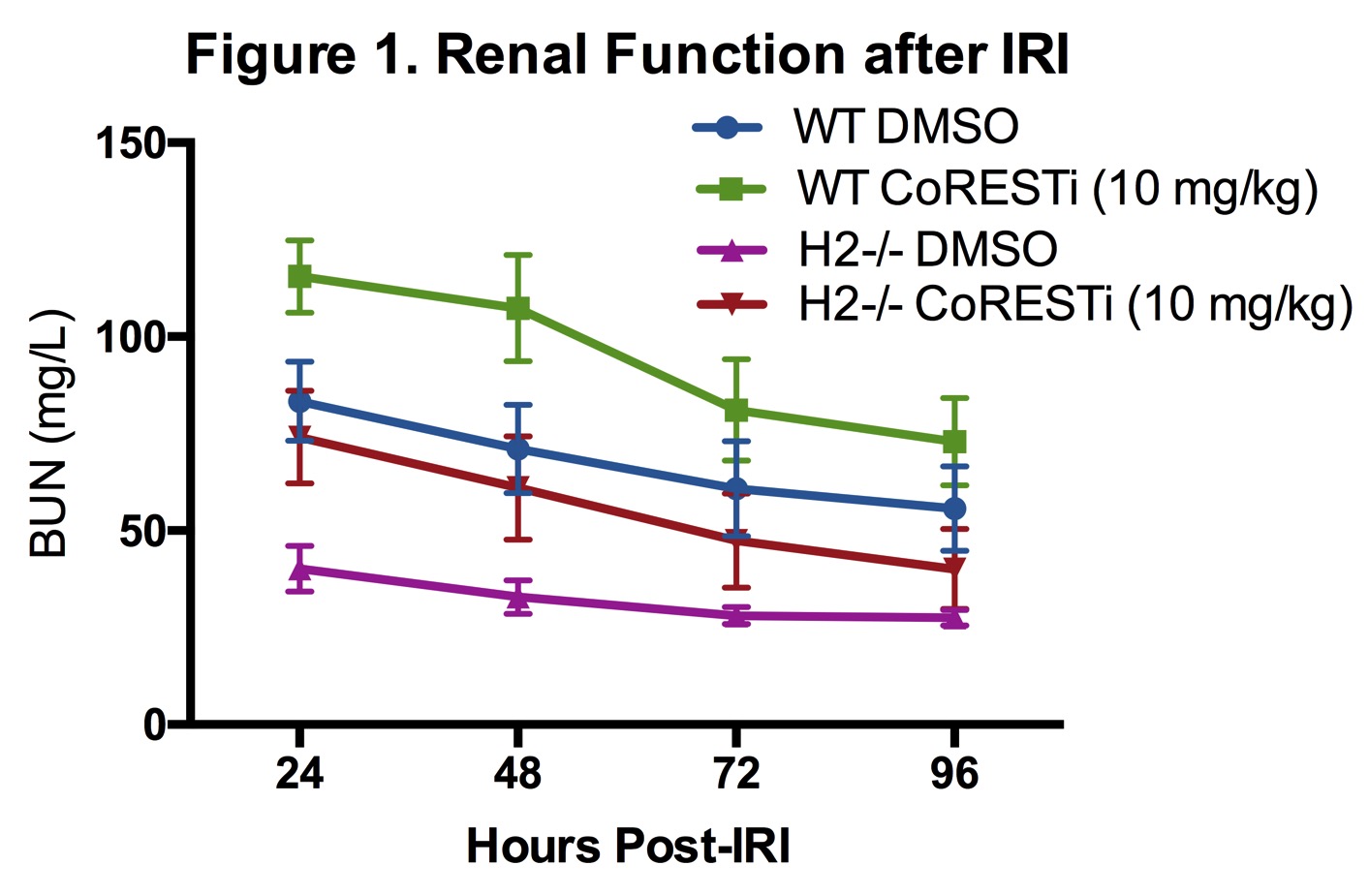
Introduction: Class I histone/protein deacetylases (HDACs) 1 and 2 bear >85% protein sequence homology and conventionally are thought to have similar function via occupancy in nuclear co-repressor complexes. Inhibition of the HDAC-axis has been shown to mitigate renal warm ischemia-reperfusion injury (IRI) in mice.
Methods: Wild type C57BL/6 (WT) and whole animal-inducible HDAC1- or 2- gene deleted mice (HDAC1-/- or HDAC2-/-) were used in this study. Standardized warm renal IRI consisted of unilateral clamping of the renal pedicle and contralateral nephrectomy. Primary renal tubular epithelial cell (RTEC) cultures were derived from each genetic strain for use in protein analysis.
Results: HDAC1-/- mice had impaired IRI tolerance compared to B6 controls with higher daily BUN levels following IRI whereas HDAC2-/- mice demonstrated profound protection from renal IRI. Protein analysis of nuclear extracts from WT, HDAC1KO, and HDAC2KO RTEC showed that deletion of either HDAC1 or 2 was associated with compensatory enhanced protein expression of the other. Pulldown experiments from RTEC extracts demonstrate that HDAC2KO leads to enhanced HDAC1 association with CoREST, NuRD and Sin3A nuclear co-repressor complexes and increased protein expression of these co-repressor components. In vivo inhibition of the CoREST complex in both WT and HDAC2-/- mice increased vulnerability to renal IRI injury and returned HDAC2-/- mice to a WT renal IRI phenotype (Figure 1).
Conclusion: HDAC1 and 2 have reciprocal effects on murine renal IRI tolerance, with HDAC1 deletion increasing vulnerability and HDAC2 deletion providing substantial protection from warm and cold ischemia. HDAC2 deletion leads to alteration in expression and possibly stability of nuclear co-repressor complexes, and its effect can be reversed with inhibition of the CoREST complex.
T. Yoo1, M. Haurani1, C. Rink1 1Ohio State University,Vascular Surgery,Columbus, OH, USA
Introduction: miRNA are short non-coding nucleotides that post-transcriptionally regulate messenger RNA. Recently, they have been discovered to be stable and amplifiable components of circulating plasma that are implicated in inflammation and remodeling. Here, we seek to identify novel circulating miRNA targets that are differentially expressed in response to vascular injury.
Methods: Rats underwent no surgery, sham left external carotid ligation, or left carotid balloon injury. Plasma samples were obtained at day 3 and day 14 after surgery. Carotid arteries were collected, sectioned and H&E stained to measure intimal hyperplasia. High resolution in vivo ultrasound was performed and analyzed to assess wall variation and intimal thickness in response to balloon angioplasty. Plasma miRNA was purified, then quantified via nCounter. Differentially expressed targets were validated with qPCR. Target Scan and Ingenuity Pathway Analysis were used to predict possible in silico targets of validated miRNA.
Results: Of the 449 genes on array, 35 were detected over background in cell free plasma samples with no indication of cellular contamination by qPCR or cell housekeeping markers. At day 3 after balloon injury there was no significant change in the miRNA profile. However, at post-injury day 14, miRNA-16 and miRNA-223 which are known to regulate proliferative and inflammatory pathways, significantly decreased compared to sham and non-operated animals (miRNA-16, Relative Expression 0.16, p = 0.001, miRNA-223 Relative Expression 0.08, p< 0.001) This corresponded to an increase in intimal thickness as detected by high-resolution, non-invasive in vivo ultrasonography and histology.
Conclusion: We have identified key circulating plasma miRNA transcriptome components that correlate to the formation of intimal hyperplasia. Identification of these targets may further elucidate miRNA regulation of vascular inflammation as well as serve as predictive biomarkers of re-stenosis following balloon injury.
S. Muralidharan1, T. Bodewes1, J. Johnson1, M. Auster1, M. Contreras1, F. W. LoGerfo1, L. Pradhan-Nabzdyk1 1Beth Israel Deaconess Medical Center,Vascular Surgery, Harvard Medical School,Boston, MA, USA
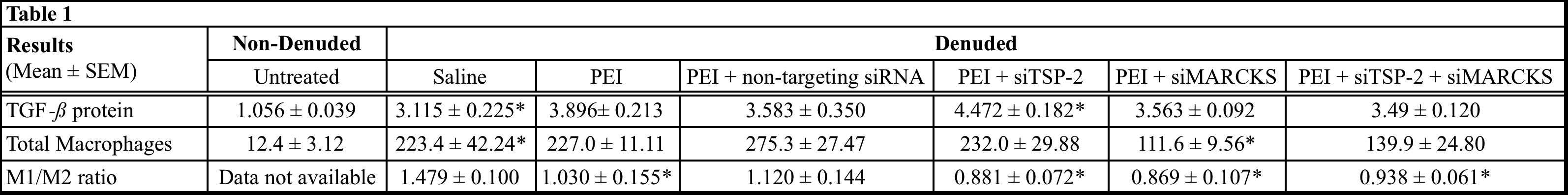
Introduction: The long-term success of vascular procedures is limited by the progression of intimal hyperplasia (IH) and subsequent loss of vessel patency. Injury at the time of implantation is known to result in dedifferentiation of vascular smooth muscle cells (VSMC) and inflammation contributing to IH; however, there is no current effective treatment that combats IH progression. Studies from our group have successfully demonstrated knock-down of IH-associated proteins, Thrombospondin-2 (TSP-2) and Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS) using siRNA in a rat model of vascular injury. The present study evaluates Transforming Growth Factor-beta (TGF-) expression and macrophage infiltration, the known effectors of IH following siRNA treatment.
Methods: One of the common carotid arteries (CCAs) was denuded in Wistar rats, using a 2F Fogarty balloon catheter. Following denudation, the artery was transfected intraluminally for 15 minutes with one of the following treatments: saline, transfection reagent Polyethylenimine (PEI), or PEI + siRNA(s) (non-targeting, siTSP-2, siMARCKS, or siTSP-2 + siMARCKS) (n=4). The denuded and non-denuded arteries were harvested at 21 days. Immunohistochemical analysis was performed to evaluate TGF- protein expression and macrophage infiltration, as well as phenotype polarization. TGF- protein expression is presented as an arbitrary score (1 to 5). Total macrophage infiltration was determined by counting all CD68+ cells and polarization by measuring pro-inflammatory M1+ (CD68+/iNOS+) and anti-inflammatory, M2+ (CD68+/CD206+) macrophages and calculating the M1/M2 ratios.
Results: Data are summarized in Table 1. Statistical significance was measured by comparing denuded saline group vs. non-denuded untreated group to validate the effect of arterial injury on TGF- expression and macrophage infiltration. For both TGF- expression and macrophage evaluation, to measure the effect of the treatment, data in the denuded treatment groups were compared to denuded saline group. Data are expressed as Mean ± SEM * = P < 0.05.
Conclusions: TSP-2 and MARCKS may play a role in modulating the macrophage polarization and their knock-down may ameliorate arterial injury-related inflammation thereby curtailing IH progression. Although the mechanism of MARCKS-mediated regulation of macrophages remains unclear, TSP-2 seems to be involved in the regulating expression of TGF-ß, a known modulator of the inflammatory response.
L. A. Scrimgeour1, B. A. Potz1, F. W. Sellke1 1Brown University School Of Medicine,Cardiothoracic Surgery,Providence, RI, USA
Introduction:
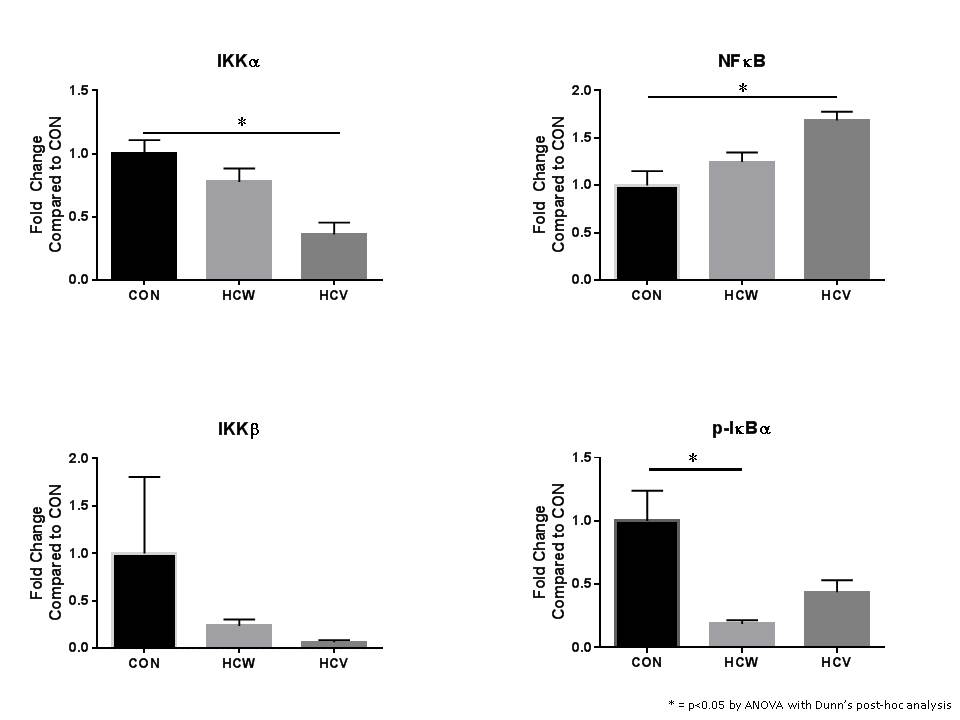
Studies suggest low alcohol consumption can be cardioprotective but it remains unclear how this effect is modulated. The Nuclear Factor kappa-B (NFκB) pathway functions to regulate the expression of genes involved in a wide variety of cellular processes involved inflammation and stress. Therefore, we used a swine model of metabolic syndrome induced by a high fat diet to investigate the effects of red wine versus vodka on NFκB signaling in chronically ischemic myocardium.
Methods:
Yorkshire swine were given a high-fat diet for four weeks, and then an ameroid constrictor was placed on the left circumflex artery. They continued on the high fat diet and were subdivided into three groups with supplementation of wine or vodka for 7 weeks; hypercholesterolemic diet alone (CON, n=8), hypercholesterolemic diet with vodka (hypercholesterolemic vodka [HCV], n=8), and hypercholesterolemic diet with wine (hypercholesterolemic wine [HCW], n=8). Animals underwent euthanasia and ischemic myocardium was harvested for analysis. This tissue was analyzed via Western blot. Protein density data were normalized to GAPDH and reported as fold-change values +/- standard error of the mean compared to control (CON) samples.
Results:
Administration of alcohol was associated with decreased expression of IKKα, IKKB and and phosphorylated IκBα in the ischemic myocardium compared to the control group. Alcohol administration was associated with an increase in NFκB in the ischemic myocardium compared to the control group [Figure 1].
Conclusions:
In the setting of myocardial ischemia, alcohol appears to modulate the NFκB pathway. This was demonstrated by the decreased expression of IKKα after alcohol administration, which decreased phosphorylation of IκBα. When less IκBα is phosphorylated, it remains bound to and inhibits NFkB from passage into the nucleus and upregulating of a variety of genes. Many of these gene products further promote inflammation and contribute to the adaptive response of tissues such as the myocardium in response to the stress of ischemia. This study provides a mechanism by which alcohol may have a protective effect on the heart.
J. T. Church1, K. B. Deatrick1, J. P. Phillips1, J. McLeod1, C. Douglas1, A. Rojas-Pena1, R. H. Bartlett1, M. L. Bocks1, G. E. Owens1 1University Of Michigan,Ann Arbor, MI, USA
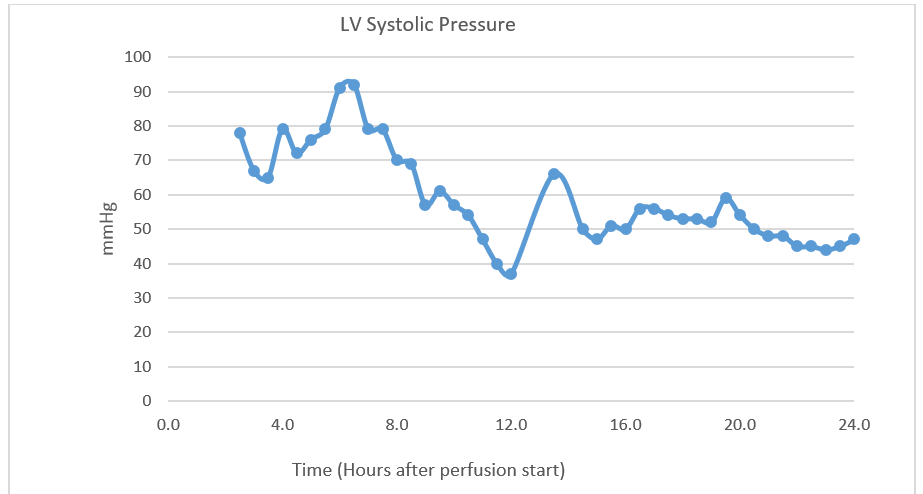
Introduction: Prolonged normothermic ex-situ heart perfusion (NEHP) could revolutionize cardiac transplantation by prolonging preservation times and allowing assessment of pre-transplant organ function. Several groups have reported preservation of porcine hearts by this method for 8-12 hours. However, neither 24-hour preservation via NEHP nor 12-hour preservation followed by successful transplantation has been reported in the literature. Here we report successful preservation of a porcine heart for 24 hours, as well as for 12 hours followed by successful transplantation, through NEHP with live paracorporeal animal plasma cross-circulation.
Methods: Porcine hearts from healthy adult swine were procured and attached to a circuit consisting of a reservoir, roller pump, and membrane oxygenator, and perfused with donor blood-based perfusate via the aortic root for antegrade coronary perfusion. A second healthy adult paracorporeal pig (PP) was placed under anesthesia with cannulation of the jugular and femoral veins for creation of a veno-venous shunt for plasma filtration. Plasma was cross-circulated between the PP and heart reservoir at 1L/hr. The primary measure of cardiac function was left ventricular (LV) systolic pressure at a set diastolic pressure of 10mmHg. In experiment one, perfusion was continued for a goal time of 24 hours. In experiment two, perfusion was continued for 12 hours, after which the perfused heart was transplanted into a healthy adult pig recipient via normal orthotopic heart transplantation techniques. Successful transplantation was defined as resumption of transplant heart contractility and the ability to wean from cardiopulmonary bypass (CPB) in the recipient pig.
Results: In experiment one, 24-hour NEHP preservation was achieved while maintaining regular electrical activity and contractions in the donor heart. Maximum left ventricular (LV) systolic pressure was 92mmHg, and was 47mmHg even after 24 hours of perfusion. The donor heart remained responsive to 0.1mg ephinephrine at the 24-hour time point, with an increase in LV systolic pressure to 196mmHg. In experiment two, the donor heart was successfully supported for 12 hours, with maximum LV systolic pressure of 97, and an LV systolic pressure of 40 at 12 hours. Following orthotopic transplantation, spontaneous cardiac contractility was achieved and the recipient was weaned off CPB, generating a blood pressure of 97/48 with inotropic and vasopressor support.
Conclusions: Successful 24-hour NEHP of a porcine heart, and 12-hour NEHP with subsequent successful transplantation, are possible using a model of NEHP that incorporates plasma cross-circulation from a live paracorporeal pig at 1L/hr.
K. Kniery1, M. DeHart1, S. Salgar1 1Madigan Army Medical Center,Clinical Investigation,Tacoma, WA, USA
Introduction:
During the global war on terrorism (2001-2008) in Iraq and Afghanistan, 737 US military service members sustained major limb amputations. Limb transplantation offers hope to improve the quality of life. We investigated whether mesenchymal stem cell (MSC) and G-CSF therapy can improve functional recovery in nerve transection-repair and limb transplant models.
Methods:
Under general anesthesia the sciatic nerve branches (tibial, peroneal and sural) were transected and repaired. In another group, syngeneic right hind limb transplantation was performed. MSCs (5×106; passage ≤6), G-CSF (50µg/kg), or Vehicle were administered topically and i.v./i.p.
Results:
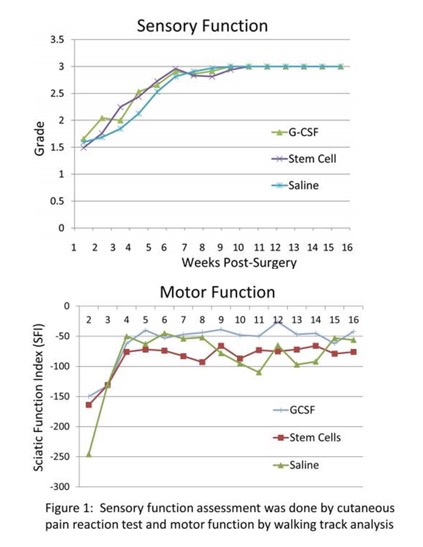
At two weeks post-nerve repair, sensory function (SF) in all groups was ~1.5 on a scale of Grade 0-3 (0=No function; 3=Normal function). By 4 weeks it was 2.2±1.0, 2.0±1.2, and 1.8±1.3 in MSC, G-CSF and Vehicle treated groups, respectively. By 10 weeks, normal SF (~3) was restored in all groups (n=8/group). The sciatic nerve function index (SFI) a measure of motor function (0=normal; -100 =nonfunctional) during 5-16 weeks was markedly improved in G-CSF (-40 to -26) compared to MSC (-93 to -66) or Vehicle (-110 to -45) group (Figure1). In limb transplants, SF recovery ranged 0.8-1.3 by 8 weeks, and 1.5-2.0 by 16 weeks post-surgery. Limb transplants (~60%) developed flexion-contractures and we were unable to calculate SFI. Fewer animals in G-CSF (37%) compared to Vehicle (66%) group developed contractures. Gastrocnemius muscle atrophy was evident in limb transplants.
Conclusion:
G-CSF and MSC therapies appear to promote sensory and motor function recovery in nerve transection-repair and limb transplant models.
L. Lin1, M. McRae1, A. MacDonald1, T. V. Brennan1 1Duke University Medical Center,Surgery,Durham, NC, USA
Introduction:
Noninvasive biomarkers of acute allograft rejection (AR) are needed to allow for early diagnosis. Serum creatinine (Cr) is an imperfect monitor of allograft dysfunction because it rises late in the process of immune injury, can be elevated for non-immunological reasons, and the magnitude of change above baseline in the setting of AR can be subtle. During AR lymphocytic invasion of allograft tissues requires the degradation of extracellular matrix. Activated CD4+ T cells express heparanase that degrade ECM and releases cleaved heparan sulfate (HS). We propose the use of plasma HS measurements to overcome the limitations of the serum Cr assay for the detection of AR.
Methods:
Serum and plasma samples from Duke University from consented kidney transplant recipients were collected and stored with approval of the Duke University IRB committee. HS concentrations in the plasma were determined by enzyme-linked immunosorbent assay. Patient groups were compared by two-tailed Student's t-test.
Results:
Patients with biopsy demonstrated AR had significantly higher plasma HS levels within 2 weeks of biopsy than patients with stable function (20.86+/-18.12 ug/mL, 95% CI=12.38-28.34 vs. 2.71+/-1.90 ug/mL, 95% CI=2.35-3.07; p<0.0001). Similarly, patients with biopsy demonstrated AR had significantly higher plasma HS levels than patients undergoing biopsy for cause, but without AR (20.86+/-18.12 ug/mL, 95% CI=12.38-28.34 vs. 3.23+/-1.61 ug/mL, 95% CI=2.26-4.20; p=0.0015) within 2 weeks of biopsy (Fig. 1A). No elevations of HS levels were found in patients with BK viremia (n=13, viral titer range 374 – 1.26×10^7 copies/mL). In comparison, elevations in serum Cr levels in patients with biopsy demonstrated AR versus patients undergoing biopsy for cause, but without AR were lesser in magnitude (5.35+/-3.18 mg/dL, 95% CI=4.09-6.60 vs. 2.64+/-1.15 mg/dL, 95% CI=1.95-3.33; p=0.0052) (Fig. 1B).
Conclusion:
Plasma HS is a sensitive biomarker of AR of kidney allografts because it is detectable prior to AR and has a greater magnitude of elevation compared with serum Cr.

D. Laan1, D. Vu1, M. Hernandez1, J. Aho1, H. Schiller1 1Mayo Clinic,General Surgery,Rochester, MN, USA
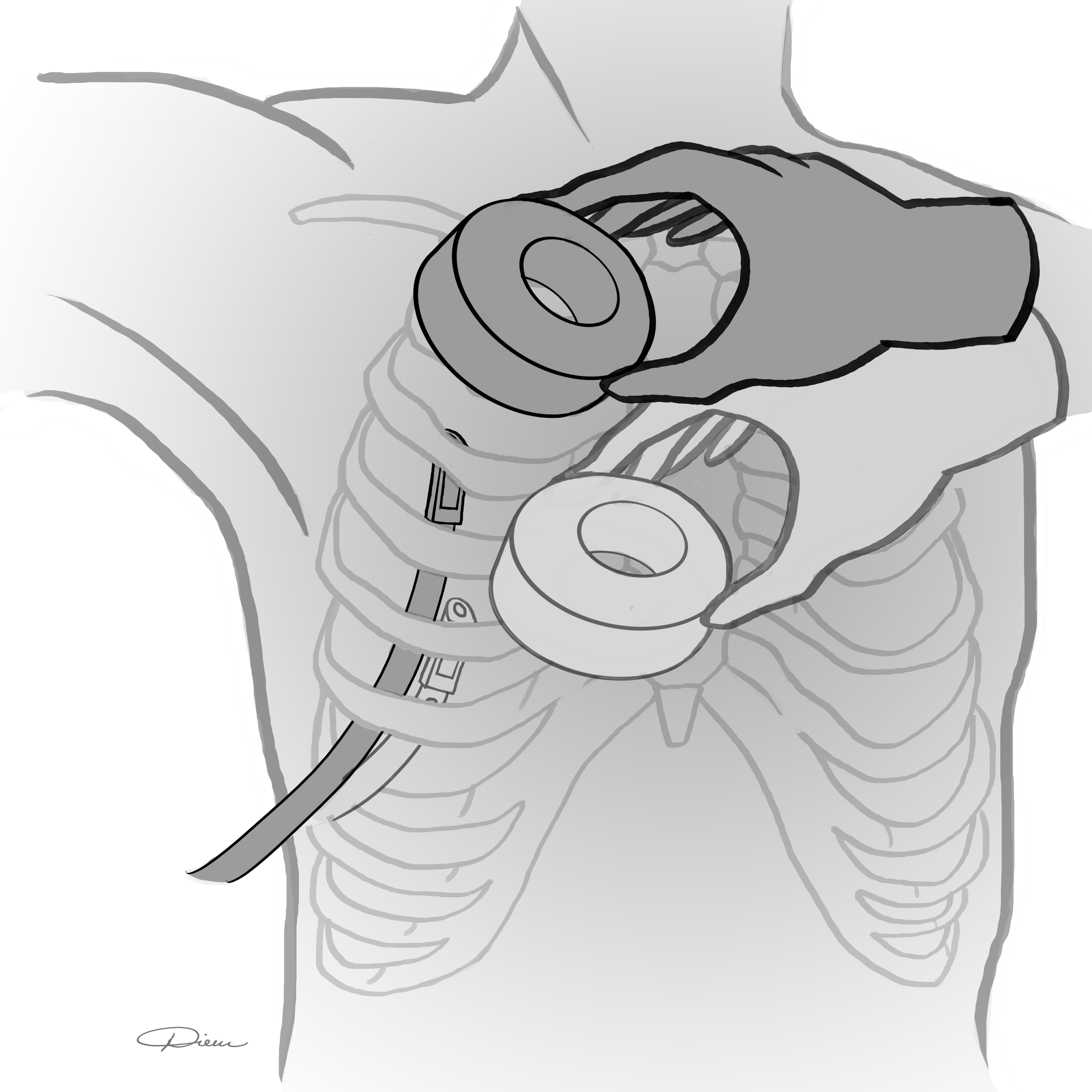
Introduction: Chest tubes can serve as life-saving adjuncts in trauma. Unfortunately, suboptimal positioning frequently complicates tube placement and can lead to impaired function. Magnets have been shown to successfully guide and direct small catheters, but this technology has never been shown to be efficacious in chest tube positioning. We sought to demonstrate, in a deceased porcine model, the utility of a 2-magnet system in directing the intra-thoracic position of a chest tube (Figure 1).
Methods: In recently deceased cross-bred domestic swine we tested magnetic positioning of a chest tube The operator held one magnet on the outside of the chest. The second magnet was introduced through a catheter to the distal tip of the chest tube. The operator was then tasked with moving the tube to distinct pre-marked intrathoracic locations under blinded conditions. This was achieved by taking advantage of the magnetic force between the two magnets. The experiment was video-recorded through an open sternotomy incision to determine success of tube positioning. Five chest tube positioning maneuvers were attempted with this system. An attempt at chest tube positioning with no magnet (standard of care) to premarked intrathoracic locations was attempted as a control.
Results:The chest tube positioning system was successful in directing a chest tube from one pre-marked location to another on 4 of 5 attempts. The system demonstrated an ability to move the tube in the cephalad-caudad axis and the anterior-posterior axis. Magnetic coupling between the 2 magnet ends was confirmed at a distance of 10cm in this model. The control chest tube with no magnet failed to navigate intrathoracically from one pre-marked location to the next with 0 of 5 attempts successful.
Conclusion:Positional flaws in chest tube placement are common. We demonstrate the 2-magnet system’s efficacy as an alternative to the traditional hand-guided method under simulated placement conditions. The pull between two magnets can be effective with up to 10cm separating magnet ends. Furthermore, we have shown with some reproducibility that a magnetic chest tube positioning system may be superior to the current standard of care technique of chest tube placement. Further study is needed to develop this emerging technology.
J. Winward1, M. E. Bowen1, H. Li1, S. H. McKellar1 1University Of Utah,Cardiothoracic Surgery,Salt Lake City, UT, USA
Introduction:
Increasing evidence indicates that abnormal energy metabolism plays a leading role in right ventricular failure (RVF), but the specific role in RVF and RV recovery (RVR) is unknown. Our analyses showed failed fatty acid oxidation (FAO) and changes in hypoxia markers in RVF and RVR. These observations led us to hypothesize that the buildup of FAO precursors was the result of insufficient mitochondrial (MT) biogenesis. Our objective was to discover how MT biogenesis is altered in RVF and RVR cardiomyoctes compared to healthy controls in a rabbit model.
Methods:
Fifteen rabbits were assigned to one of three groups—control, RVF, and RVR—and RV tissue samples were taken from each rabbit’s RV. MT gene expression and transcriptional variance were measured via RT-PCR and RNA sequencing. Immunoblotting of MT biogenesis activators was also performed.
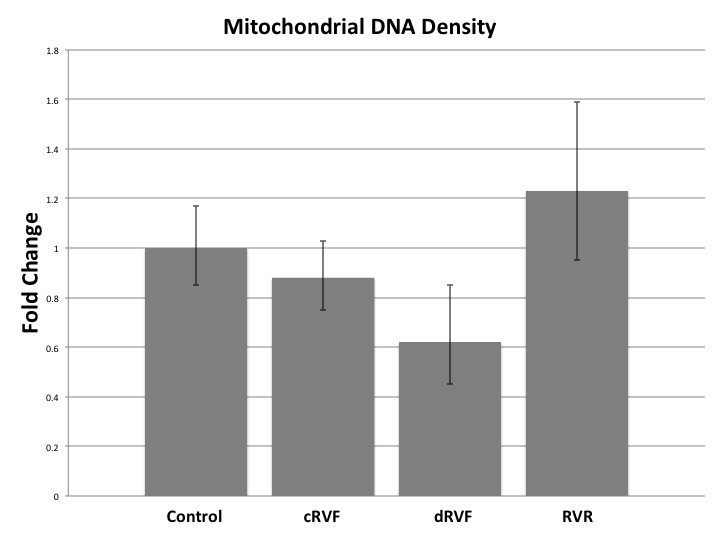
Results:
MT DNA density decreased during RVF with fold changes of RVF=0.75 (p=0.22) returning to RVR=1.06 (p=0.84). Further division of the RVF group into compensated RVF (cRVF) and decompensated RVF (dRVF) revealed that MT density was qualitatively greater in cRVF (FC=0.88, p=0.58) than in dRVF (FC=0.62, p=0.26). RNA sequencing revealed significantly increased transcription of HIF in RVF when compared to the control (FC=0.93; p<0.04) and RVR (FC=0.90; p<0.03). However, the rate of transcription of downstream activators of MT biogenesis (i.e. PGC-1-α, NRF-1, NRF-2, Akt3, Perm-1, TFAM, VEGF, and AMPK) was not significantly altered between the groups. Despite its unaltered transcription, immunoblotting of activated AMPK revealed a significant fold increase in RVF (1.50) and RVR (1.61) compared to the control (1.06) (p=0.01).
Conclusion:
Our data qualitatively suggest that MT DNA density decreases in a manner directly proportion to the severity of RVF, and that it increases in RVR. Other studies show similar findings in human subjects. Additionally, the increased transcription of HIF in the RVF samples leads us to hypothesize that hypoxia plays a significant role in the MT pathogenesis of RVF. The discrepancy between the rate of transcription and the enzyme activation of AMPK, in conjunction with the altered MT DNA density in RVF and RVR despite the unchanged rate of transcription of upstream MT biogenesis regulatory enzymes, leads us to hypothesize that the functionality rather than the quantity of MT biogenesis regulators is leading to the variance in MT DNA density, as seen with the increased levels of the activated AMPK protein in RVF and RVR, despite its unaltered rates of transcription. Further research investigating the alterations of MT biogenesis markers at the transcriptional and translational level, and hypoxia’s role therein, is warranted.
A. AlKukhun1, A. Bertacco1,2, G. Caturegli1, F. D’Amico1,2, R. Morotti1, M. I. Rodriguez1, D. C. Mulligan1, J. P. Geibel1 1Yale University School Of Medicine,Surgery,New Haven, CT, USA 2Padova University,HPB And Liver Transplant,Padova, VENETO, Italy
Introduction: The small intestine remains one of the most sensitive organs to ischemic injury prior to transplantation. In the present study, we assessed in a rat model the optimal preservation temperature for intestinal grafts by comparing normothermic to hypothermic perfusion preservation.
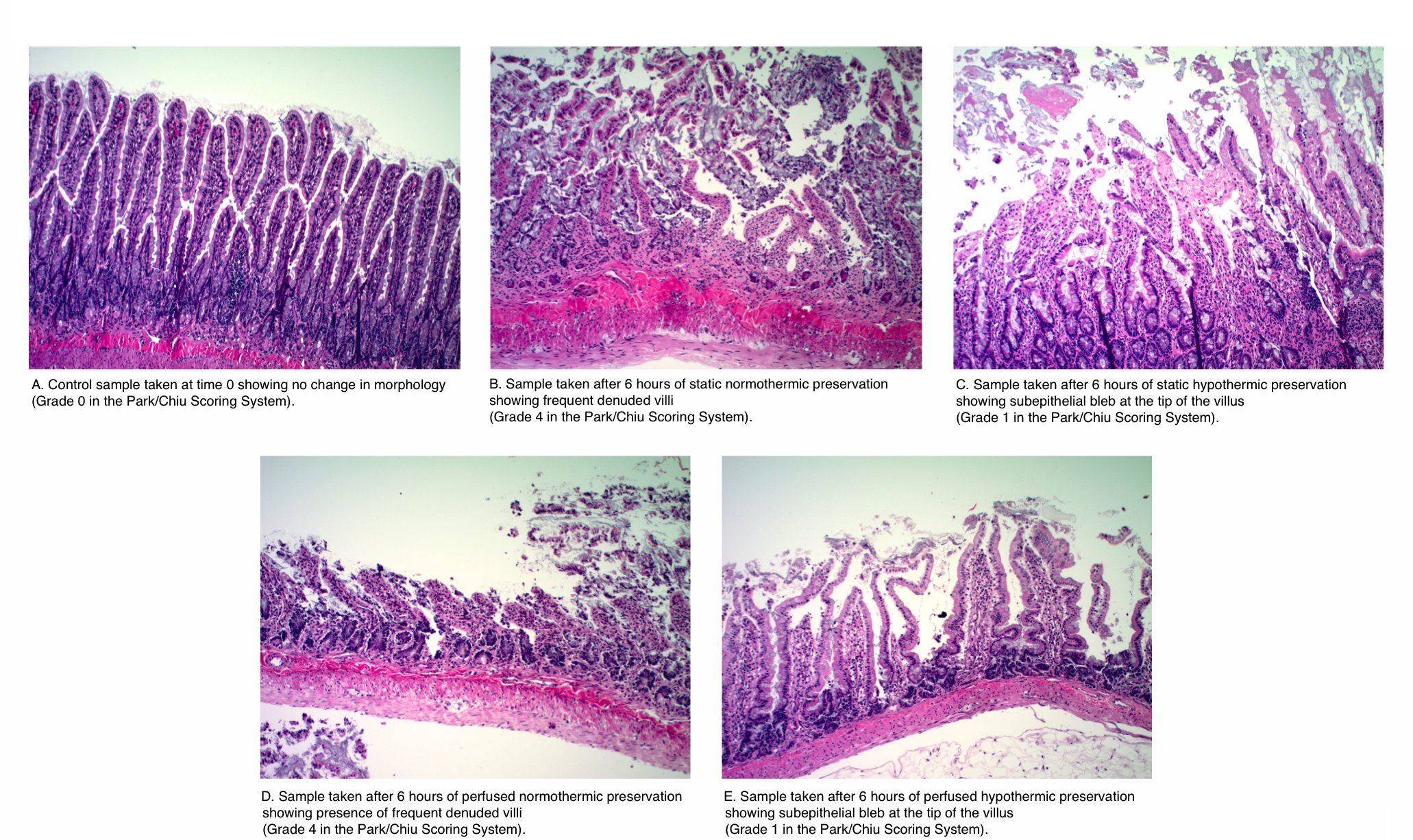
Methods: Small intestine segments were procured from rats and connected to customized chambers and a perfusion system. Paired intestinal segments were perfused in the luminal and vascular side with University of Wisconsin Solution at two different temperatures (37 and 4 Celsius) over 6 hours. Intestinal tissue samples were processed and evaluated by a blinded pathologist using the Park/Chiu Scoring System for Grading Intestinal Ischemia.
Results: The histopathological analysis showed that continuous perfusion of the intestinal segments at 4 Celsius better preserves the small intestinal segment in comparison to perfusion at 37 Celsius at 6 hours. The differences among the means (hypothermic: 1.67 vs. normothermic: 3.5) in the scoring was significant at an alpha level of 0.05 (p-value=0.0048). The attached figure displays the pathological analysis of the samples.
Conclusion: In conclusion, continuous hypothermic perfusion of small intestinal grafts with University of Wisconsin solution resulted in improved preservation of the intestinal tissue in comparison to continuous normothermic preservation. These results suggest that continuous perfusion at normothermic conditions shows no advantage to continuous hypothermic perfusion as judged by pathological evaluation.
J. Seal1, H. Bohorquez1, D. Bruce1, E. Bugeaud1, I. Carmody1, A. Cohen1, G. Loss1, J. Culver2,3, C. Petucci2,3, S. Gardell2,3 1Ochsner Clinic,Multi-Organ Transplant,New Orleans, LA, USA 2Sanford Burnham Prebys Medical Discovery Institute,Orlando, FL, USA 3Southeast Center For Integrated Metabolomics,Gainesville, FL, USA
Introduction: Donation-after-circulatory death (DCD) donors constitute an important opportunity to expand the donor pool for liver transplantation. Utilization of DCD livers is limited by the duration of donor warm ischemia (WIT) from withdrawal of support to hypothermic preservation. Most transplant centers adhere to a fixed limit of donor WIT for acceptance of DCD liver for transplant. Targeted metabolomic analysis generates a “snapshot” of the physiologic state of tissue by measuring the abundance of specific metabolites. A metabolomic approach to donor liver assessment may offer insight into key changes liver allograft physiology and offer a more precise approach to guide organ selection.
Methods: We applied a metabolomics approach to comprehensively quantify mediators of central energy metabolism in DCD (N=10) vs standard brain dead donors (DBD, N=14). Donor liver biopsies were prospectively obtained at the end of cold storage prior to implantation, flash frozen and biobanked at -70 C storage. Metabolomic analyses were performed through the Southeast Center for Integrated Metabolomics (SECIM). Lyophylized liver tissue samples were reconstituted and analyzed using liquid chromatography (LC) and mass spectroscopy (MS) to quantify amino acids (AA), organic acids, pyridine and adenine nucleotides and acetyl-/malonyl-CoAs using isotope-labeled internal standards.
Results: The mean donor WIT in the DCD group was 20.5±7.8 minutes. There was no differences in cold ischemia time for DCD and DBD groups (4.8 vs 4.3 hrs, P=0.42) and both groups had 100% patient and graft survival at 1 year. Concentrations of ATP and ADP were significantly lower in the DCD group (P=0.021). Acetyl-CoA, a key mediator between glycolysis and Krebs cycle, was also markedly decreased in DCD livers (-37% P=0.009). With the exception of glutamine, all AA were significantly higher in DCD livers compared to DBD grafts , ranging from 44.8% increase for glycine (P=0.022) to 104.2% increase for tyrosine (P=0.001). Tyrosine is a non-essential AA that is both keto- and glucogenic. All amino acids that displayed significant increase in DCD livers can replenish TCA cycle intermediates through anaplerotic reactions (salvage pathways). We did not observe significant differences in organic acid levels between DCD and DBD donors, suggesting preservation of TCA cycle function through protein catabolism and anaplerosis. Significant increase in NMN, a precursor to NAD, in the DCD groups suggests a potential “bottleneck” in pyridine nucleotide metabolism at the NMN adenyltransferase enzyme.
Conclusion: Using a targeted metabolomics approach, we have identified distinct alterations in energy metabolism in DCD livers compared with DBD controls. Expanded applications of targeted metabolomics offers the potential to develop biomarkers to augment DCD liver allograft evaluation and selection for transplantation.
J. Johnson1, T. C. Bodewes1, S. Muralidharan1, M. Auster1, M. Contreras1, F. W. LoGerfo1, L. Pradhan-Nabzdyk1 1Beth Israel Deaconess Medical Center,Vascular Surgery, Harvard Medical School,Boston, MA, USA
Introduction: Long-term patency rates following vascular interventions remain poor due to the formation of intimal hyperplasia (IH). Currently, no preventive treatment is available to attenuate long-term failure. This study evaluates the ability to inhibit expression of Thrombospondin-2 (TSP-2) and Myristoylated Alanine-Rich C-Kinase Substrate (MARCKS), the IH-associated proteins identified by our group, using commercially available siRNAs in a rat model of IH.
Methods: One of the carotid arteries of Wistar rats were denuded using a 2F Fogarty balloon catheter. Following denudation, the artery was transfected intraluminally for 15 minutes with one of the following treatments: saline, transfection reagent Polyethylenimine (PEI), or PEI + siRNA(s) (non-targeting, siTSP-2, siMARCKS, or siTSP-2 + siMARCKS) (n=6). Denuded and non-denuded contralateral arteries were harvested at 24 hours or 21 days for analysis. TSP-2 and MARCKS gene expression were determined using q-RT-PCR. TSP-2 and MARCKS protein expression and cell proliferation were analyzed using immunohistochemistry. mRNA expression is presented as fold change over the control (either non-denuded untreated or denuded saline treated) . Protein expression is presented as an arbitrary score (1 to 5). Cell proliferation is presented as no. of Ki+ cells/mm2.
Results: Data are presented in Table 1. Statistical significance was measured by comparing denuded saline group vs. non-denuded untreated group to validate the effect of arterial injury on target gene expression and cellular proliferation (represented by †). Data in the denuded treatment groups were compared to denuded saline group to measure the effect of the treatment on denudation-related target gene expression and cellular proliferation (represented by *). Data are expressed as Mean±SD. * = P < 0.05.
Conclusion: The carotid artery balloon injury in rats up-regulated the expression of TSP-2 and MARCKS proteins, previously identified in large animal models of IH and respective siRNAs reduced their expression. This model can serve to investigate siRNA-based therapies targeting proteins for IH prevention. Preliminary results suggest that combination siRNA treatment targeting IH-associated proteins could be a feasible approach to curtail IH.
C. T. Holmes1, N. Clarke1, L. Powell1, M. E. Jessen1, M. Peltz1 1University Of Texas Southwestern Medical Center,Department Of Cardiovascular And Thoracic Surgery,Dallas, TX, USA
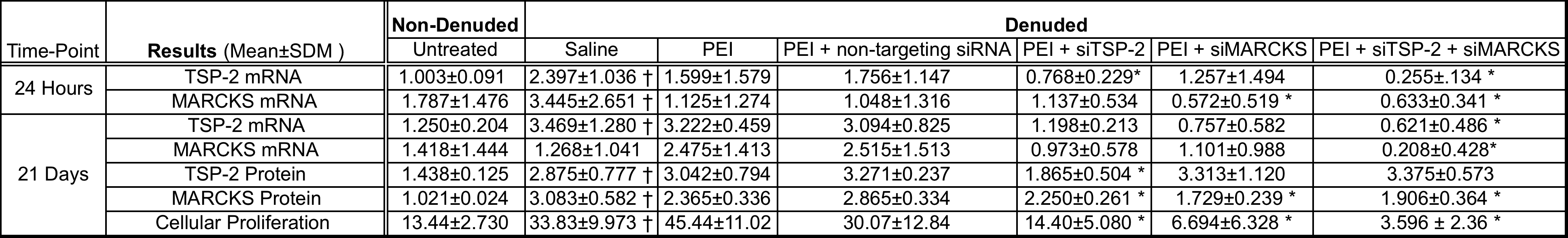
Introduction: Coronary artery bypass grafting remains the standard of care for treatment of multivessel coronary artery disease, particularly in diabetic patients. The influence of diabetic therapies on myocardial substrate selection under these conditions are unknown but may be important to ensure optimal outcomes after cardiac surgery. We hypothesized metformin and insulin alter myocardial substrate selection during cardiac surgery and may effect reperfusion cardiac function.
Methods:
Groups of rat hearts (n=4 per group) were perfused under 3 conditions: Normokalemia, Cardioplegia or Bypass. Normokalemia groups were perfused with Krebs-Heinseleit buffer in the presence of no additives (Control), 500mM metformin (Metformin), 10 units/L insulin (Insulin), or both insulin and metformin (Metformin + Insulin). Cardioplegia animals were perfused with the same additives for 30 minutes with potassium modified buffer (20mM) to simulate cardioplegic arrest. Bypass groups containing the same additives were treated with three 22-minute ischemic intervals followed by a 3-minute interval of perfusion with cardioplegia buffer and 30 minutes of normokalemic reperfusion to simulate conditions encountered during coronary artery bypass grafting. Perfusion buffer with physiological concentrations of fatty acids (.35mM), ketones (.17mM), lactate (1.2mM), pyruvate (.12mM), and glucose (5.5mM) with different Carbon-13 (13C) labelling patterns was used for all conditions. 13C NMR spectra were obtained. Fractional substrate oxidation was determined by glutamate isotopomer analysis . Myocardial efficiency (rate*pressure/oxygen consumption) was measured in Bypass groups. Groups were compared by one-way analysis of variance or t-tests.
Results:
During cardioplegia, fatty acid oxidation was decreased for all additives. Ketone oxidation in this condition was increased with all additives except for metformin. Fatty acid oxidation was increased in all groups with a corresponding decrease in endogenous substrate oxidation when additive was included in the perfusion buffer. See Figure. Myocardial efficiency was not different for each additive compared to the stabilization period.
Conclusion:
Conditions encountered during cardiac surgery result in alterations in myocardial substrate oxidation profiles resulting in a reduction in fatty acid oxidation during potassium cardioplegia but increased fatty acid oxidation after reperfusion. These alterations in substrate oxidation did not affect myocardial efficiency in otherwise normal hearts.
K. A. Kelley1, R. Ruhl3, S. Rana2, C. R. Thomas2, S. Anand2,3, V. L. Tsikitis1 1Oregon Health And Sciences University,Department Of Surgery,Portland, OREGON, USA 2Oregon Health And Sciences University,Department Of Radiation Medicine,Portland, OREGON, USA 3Oregon Health And Sciences University,Department Of Cell, Developmental, Cancer Biology,Portland, OREGON, USA
Introduction:
Chemoradiotherapy (CRT) response is an independent predictor of overall survival in locally advanced rectal cancer, highlighting the importance of improving CRT response rates. It is known that several tumor intrinsic factors govern responses to radiation therapy. Emerging evidence suggests that microRNAs (miRs) modulate gene expression profiles in response to radiation.
Methods:
microRNA profiles were examined and analyzed in approximately forty rectal cancer patients that had either a pathological partial response (PR) or no response (NR). Using Nanostring technology, a miR profiling platform, miRNAs significantly down and up regulated were identified in FFPE biopsy specimens. mRNA differentially expressed was followed by in vitro radiation models and tumor sphere assays. Graph 1.
Results:
miR-451a was among the most upregulated miRs in the PR group whereas miR-203 was among the most downregulated miR. Inhibition of miR-451a in HCT-116 significantly increased survival in response to a 2 Gy dose of radiation; conversely, transfection of a miR-451 mimic significantly decreased cell survival, with more than a 12-fold decrease when combined with a 5 Gy dose fraction. Consistent with these results, miR-451a inhibition increased proliferation in 2D as well as in a tumor sphere assay, whereas transfections of a miR-451a mimic robustly decreased proliferation. In contrast to miR-451a, miR-203 was downregulated in patients with a partial response to CRT. Inhibition of miR-203 increased apoptosis in combination with a 5 Gy dose fraction.
Conclusion:
A gain of miR-451a and an inhibition of miR-203 in tumor cell lines enhanced cell death and decreased survival in combination with radiation. In this context, we hypothesize that differential expression of miRs regulates rectal cancer radiation sensitivity and therefore can be used as a biomarker to predict therapeutic responses to radiation therapy.
K. M. Sokolowski1, S. Kunnimalaiyaan1, T. C. Gamblin1, M. Kunnimalaiyaan1 1Medical College Of Wisconsin,Surgical Oncology/Surgery,Milwaukee, WI, USA
Introduction:
Cholangiocarcinoma (CCA) is characterized by poor prognosis and therapeutic inefficacy. The current standard of practice for advanced CCA is systemic chemotherapy with gemcitabine and cisplatin. However, a limited survival benefit along with increasing resistance has ushered in a renaissance in alternative approaches. Increased understanding and application of new technologies has led to the identification of more pathway-focused treatment strategies. The glycogen synthase kinase-3 (GSK-3) pathway is a potential therapeutic target as it is overexpressed in various cancer types including CCA. However, the role of targeting GSK-3 in CCA progression remains elusive. We hypothesize that GSK-3 stabilizes cellular growth thereby promoting proliferation and with subsequent inhibition, leading to a reduction in cellular growth in vitro. For this, we have used a clinically well-characterized GSK-3 inhibitor, LY2090314.
Methods:
The effects of LY2090314 on CCA cell lines (CCLP-1 and SG-231) were assessed by MTT and colonogenic assay. Cell lysates were analyzed via Western blotting for pro-apoptotic, anti-apoptotic and cell cycle proteins. Apoptotic induction was further evaluated through caspase-glo 3/7 assay. Mechanism of LY2090314 administration on CCA cellular proliferation was also examined.
Results:
Increasing LY2090314 treatment (0μM – 20μM) had a significant dose and time-dependent growth reduction (p<0.01) compared to control. The concentration at which 50%growth inhibition (IC50) was observed in vitro following 96hr treatment for CCLP-1 and SG-231 is 13.7µM and 9µM respectively. Similar concentrations inhibited colony formation in both CCA cell lines. Western analysis indicated that growth suppression was due to apoptosis as well as cell cycle arrest as evidenced by increased expression of pro-apoptotic (cleaved PARP and cleaved caspase-3), reduced anti-apoptotic (survivin) proteins and mitigation of cell cycle arrest proteins (p21 and cyclin D1). Functionally, this was confirmed by an increase in caspase activity. Importantly, LY2090314 treatment reduced the expression levels of only GSK-3β in CCA cell lines.
Conclusion:
LY2090314, a GSK-3 inhibitor, effectively reduces CCA growth in vitro and appears through reduction of GSK-3β. To our knowledge, this is the first study on the effect of LY2090314 in cholangiocarcinoma cell lines in vitro and provides rationale for further preclinical analysis.
S. G. Patel1, L. Li1, A. Maas1, R. Ghukasyan1, J. Williams1, P. Toste1, A. Nguyen1, I. Elliott1, N. Wu1, T. Donahue1 1University Of California Los Angeles,Surgery,Los Angeles, CA, USA
Introduction:
We have demonstrated that pancreatic cancer (PDAC) tumor associated fibroblasts (TAFs) support pancreatic cell growth and invasion from a pro-inflammatory, DNA damage (DDR) mediated pathway. This pathway referred to as the senescence associated secretory phenotype (SASP) requires both stress kinases and Nfkb signaling for maximum signal production after stimulus. Within the tumor microenvironment, genotoxic chemotherapies can stimulate the stromal cells to produce factors that promote tumor cell fitness. Contrary to the prevailing model, we have identified that JNK isoforms are cell-type specific and that both JNK1 and JNK3 can transduce this inflammatory pathway in the tumor microenvironment. We additionally have found that not all of the SASP is regulated by Nfkb, but rather a key mediator, IL-1 alpha, can be expressed in its absence.
Methods:
Immortalized pancreatic cancer tumor associated fibroblasts (Logsden Lab) as well as Hela cells were used to create both gene knockouts as well as stable knockdowns for JNK1, JNK3 and p65 (Nfkb signaling). SASP induction was verified after a DNA damage response using RTPCR. Small molecules specific to JNK isoforms were used to verify genetically manipulated cell lines in WT cells.
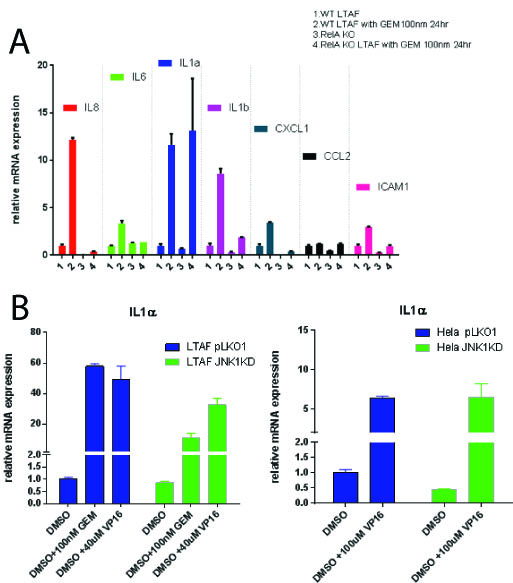
Results:
Gemcitabine treatment (100 nm) of PDAC TAFs induced a pro-inflammatory gene expression program including statistically significant (p < 0.05) upregulation of a number of cytokines: including IL-6, IL-8, IL-1 alpha and beta, CXCL1, CCL2 and ICAM1. Homozygous knockout TAFs for p65 (TAF p65 -/-) stops the induction of these all genes tested (p<0.05) except IL-1 alpha (p = 1) [Figure 1A]. Nfkb (p65) is required for upregulation of IL-1 alpha in the presence of recombinant (10 ug/mL) TNF-alpha or IL-1 alpha (p <0.05). We found that TAFs do not express JNK3, but rather JNK1 and 2. We identified using a combination of shRNAs to JNK1 and JNK3 as well as small molecule inhibitors to JNK1/2 that TAFs require JNK1 for upregulation of IL-1 alpha after a DDR (p<0.05) [Fig 1B]. This implies that in the tumor microenvironment, the SASP is propagated by specific stress kinases expressed.
Conclusion:
Previous studies have attributed the SASP to be critically dependent on Nfkb signaling as well as amplified in culture with JNK3. We identify that some elements of the SASP are not dependent on Nfkb signaling. Furthermore, we find that JNK3 is not a ubiquitous stress kinase in this response, but rather JNK isoforms are cell-type restricted in expression. These findings highlight the complexity of the pro-inflammatory signaling within the tumor microenvironment and suggest a more careful analysis of cell and tissue specific gene expression is needed for developing optimal treatment strategies.
M. Nakajima1, Y. Miura1, T. Ando1, K. Yuza1, J. Tsuchida1, Y. Tajima1, M. Abe2, K. Sakimura2, T. Wakai1, M. Nagahashi1 1Niigata University Graduate School Of Medical And Dental Sciences,Division Of Digestive And General Surgery,Niigata City, , Japan 2Brain Research Institute, Niigata University,Department Of Cellular Neurobiology,Niigata City, , Japan
Introduction: Pancreatic cancer is one of the most lethal diseases known, and it is important to develop new therapeutic agents. Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator that regulates cell survival, migration, immmune cell recruitment, angiogenesis and lymphangiogenesis, which are all factors involved in cancer progression. S1P, which functions intra- and extracellularly, is generated inside the cell by two sphingosine kinases (SphK1 and SphK2). We have reported that SphK1 plays an important role in S1P secretion (J Biol Chem 2010) and cancer progression (Cancer Res 2012, J Surg Res 2016), and that SphK2 has a unique role in regulating cellular functions in the liver (Hepatology 2015). Little is known, however, about the role of SphK1 and SphK2 in pancreatic cancer progression. The aim of this study is to investigate the role of SphK1 and SphK2 in pancreatic cancer progression using SphK-knockout (KO) cells generated by CRISPR/Cas9 technology.
Methods: We generated Pan02 murine pancreatic cancer cell lines with a CRISPR/Cas9 mediated targeted deletion of the SphK1 or SphK2 gene. Western blotting and RT-qPCR determined the expression levels of SphKs in these cell lines. To investigate the role of SphK1 or SphK2 in cellular proliferation, we assessed cell growth by a spectrophotometric technique using the water-soluble tetrazolium salt, WST-8. Cell migration was measured by an in vitro scratch assay.
Results: We confirmed the knockout of SphK1 or SphK2 in Pan02 pancreatic cancer cells by western blotting and RT-qPCR. SphK2 KO cells were significantly less proliferative than wild type (WT) cells. Unexpectedly, SphK1 KO cells were significantly more proliferative than WT cells. The in vitro scratch assay indicated that SphK2 KO cells were less migratory than WT cells, and that SphK1 KO cells had greater migratory ability than WT cells. These results indicate that S1P produced by SphK2, rather than by SphK1, may have important roles in proliferation and migratory behavior in pancreatic cancer cell lines.
Conclusion: Our findings indicate that S1P produced by SphK2, rather than by SphK1, promotes pancreatic cancer cell proliferation and migration. Targeting SphK2 may be a potential strategy for pancreatic cancer treatment. Further studies that include in vivo models are needed to explore this therapeutic possibility.
V. N. Nfonsam1, J. Koblinski1, J. Jandova1 1University Of Arizona,Surgery,Tucson, AZ, USA
Introduction: Although the overall incidence of colon cancer (CC) has steadily declined over the last three decades, the incidence has increased in patients younger than 50. The etiology of early-onset (EO) CC is not fully understood. COMP gene has been shown to confer tumor aggressiveness in pancreatic cancer. The aim of this study was to elucidate gene expression patterns in EOCC and show its uniqueness compared to late-onset (LO) disease.
Methods: Two cohorts of patients with sporadic CC were identified. Tumors and matching noninvolved tissues from six EOCC patients (<50) and six late-onset colon cancers (LOCC) patients (>65) were obtained from pathology archives. De-paraffinized tissues were macro-dissected from FFPE sections, RNA isolated, and used for expression profiling of 770 cancer-related genes representing 13 canonical pathways. Survival analysis was performed using the cBioPortal for cancer genomics using 382 CRC patients from the TCGA Provisional database.
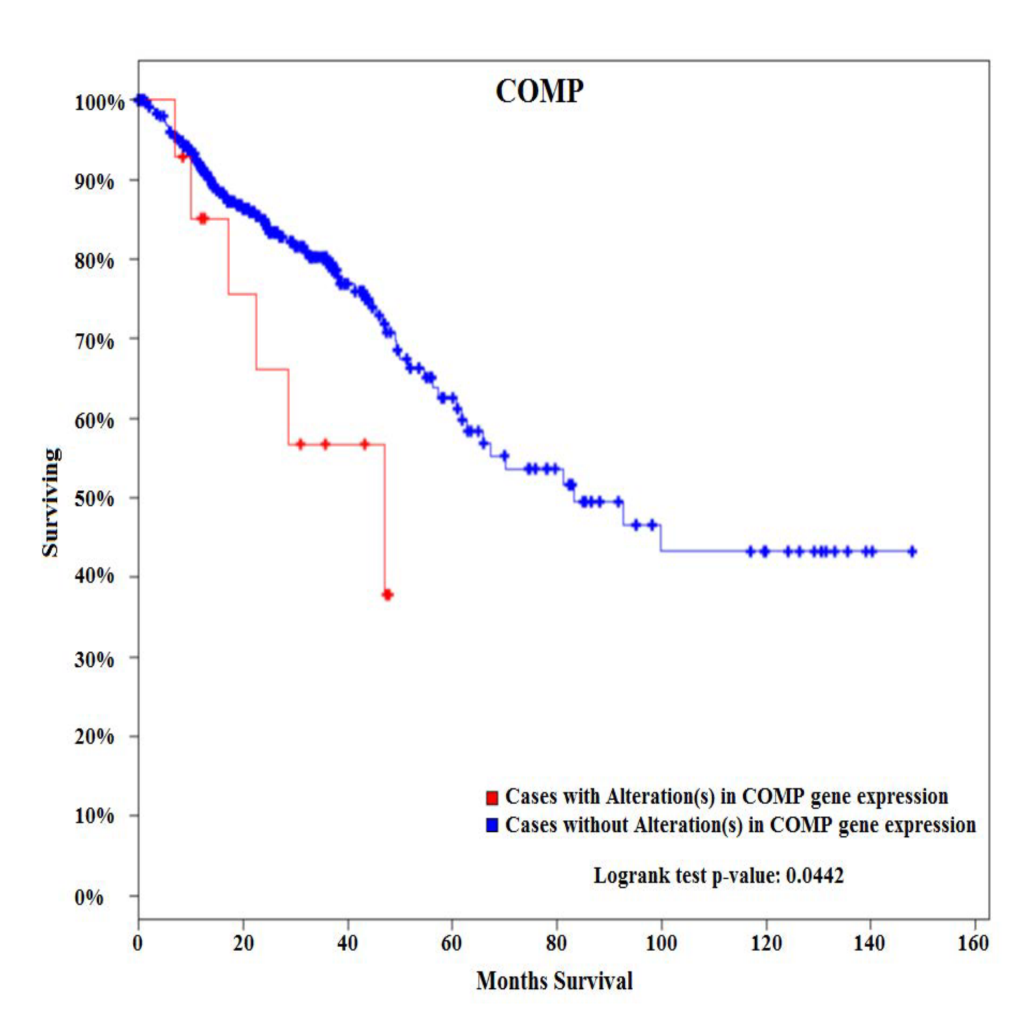
Results:Among 770 genes assayed, changes in expression levels of 93 genes were statistically significant between EOCC and matching noninvolved tissues. There were also significant differences in expression levels of 118 genes between LOCC and matching noninvolved tissues. Detailed comparative gene expression analysis between EOCC and LOCC normalized to their matching noninvolved tissues revealed that changes in expression of 88 genes were unique to EOCC using the cutoff criteria of expression levels difference >2 fold and P value <0.01. From these differentially expressed genes specific to EOCC, 28 genes were upregulated and 60 genes downregulated. At the pathway level, PI3K-AKT signaling was the most deregulated pathway in EOCC and cell cycle in LOCC. COMP glycoprotein was one of the genes that were uniquely over-expressed in EOCC. Survival analysis of 382 patients with CRC tumors using the cBioPortal for cancer genomics showed that CRC patients with alterations (all of them with up-regulated COMP) in COMP expression present with poorer overall survival compared to patients without alterations in COMP expression.
Conclusion:
These results suggest that sporadic EOCC is characterized by distinct molecular events compared to LOCC. In addition, COMP glycoprotein is associated with poor overall survival. COMP gene can potentially serve as a novel biomarker associated with EOCC as COMP glycoproteins could be detected in serum and urine.