C. J. Corbett1, J. D. Predina3,5,6, A. D. Newton2, M. Shin2, L. Sulyok2, L. Xia2, P. S. Low7,8, S. Singhal1,2,3,4 1University Of Pennsylvania,Perelman School Of Medicine,Philadelphia, PA, USA 2Hospital Of The University Of Pennsylvania,Department Of Surgery,Philadelphia, PA, USA 3Hospital Of The University Of Pennsylvania,Center For Precision Surgery,Philadelphia, PA, USA 4Hospital Of The University Of Pennsylvania,Department Of Thoracic Surgery,Philadelphia, PA, USA 5Harvard School Of Medicine,Brookline, MA, USA 6Massachusetts General Hospital,Department Of Surgery,Boston, MA, USA 7Purdue University,Department Of Chemistry,West Lafayette, IN, USA 8On Target Laboratories,West Lafayette, IN, USA
Introduction:
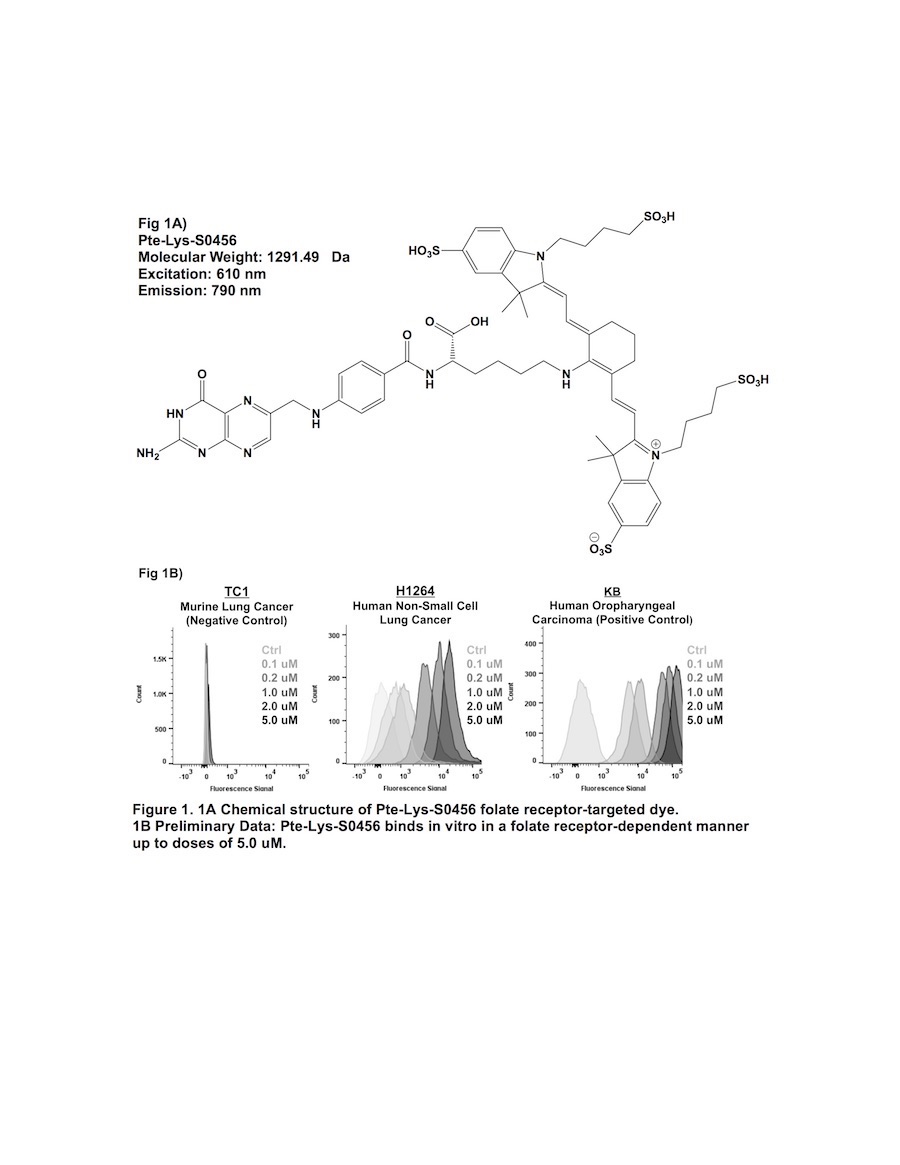
Previous attempts of folate receptor-targeted intraoperative molecular imaging (IMI) of pulmonary adenocarcinomas have been successful, but remain limited by photon-scatter and high levels of tissue reflection. One approach to overcome these limitations is by incorporating fluorophores displaying increased Stokes shifts (greater than 100 nm). In this study, we describe optical properties and preclinical data involving a third-generation folate receptor-targeted probe, Pte-Lys-S0456, that displays a Stokes shift measuring 180 nm.
Methods:
Excitation and emission spectra for Pte-Lys-S0456 were obtained with 10 nM aliquots measured with a luminometer. Next, several murine and human NSCLC models were co-cultured in variable concentrations of Pte-Lys-S0456 ranging from 100 nM to 10 uM for 2 hours. Following washout, fluorescence was assessed with flow cytometry and fluorescence microscopy. Fluorescent patterns were quantified and compared to previous folate receptor-targeted drugs (Folate-FITC and Folate-S0456). Following in vitro characterization, in vivo feasibility of systemc Pte-Lys-S0456 was tested using a small animal flank tumor model of cancer surgery (n=15).
Results:
The peak excitation and emission wavelengths of Pte-Lys-S0456 were found to be 610 nm and 790 nm (Stokes shift of 180 nm). NSCLC models avidly bound Pte-Lys-S0456 in vitro in a folate receptor-dependent manner. Fluorescent signal of tumor cells co-cultured with Pte-Lys-S0456 resulted in increases in fluorescence up to a concentration of 5 uM (p < 0.001). Higher concentrations of Pte-Lys-S0456 did not improve tumor fluorescence. Upon fluorescent microscopic evaluation of tumor models, we observed strong cell membrane binding of Pte-Lys-S0456 in all groups. In cells exposed to the highest concentration, we observed internalization of Pte-Lys-S0456. When compared to Folate-FITC and Folate-S0456, we observed improved signal-to-background signal due to decreased tissue reflection and photon scatter.
Conclusion:
Pte-Lys-S0456 is a novel folate receptor-targeted optical contrast agent that binds NSCLC in a folate receptor-dependent manner. Improved optical properties associated with Pte-Lys-S0456 result in higher in vivo signal-to-background ratios, and thus may provide a more reliable agent for folate receptor-targeted IMI of human NSCLC.