D. Sood1, A. Cazes1, D. Jaquish1, E. Mose1, R. French1, A. M. Lowy1 1University Of California – San Diego,Department Of Surgery, Division Of Surgical Oncology, Moores Cancer Center, La Jolla, CA, USA
Introduction:
RON, a receptor tyrosine kinase and c-Met proto-oncogene homolog, is over-expressed in pancreatic cancer relative to normal tissue. RON’s function in normal biology is to curtail the inflammatory response, such as in the terminal stages of wound healing. It does this in large part by regulating the transition of macrophages from a pro-inflammatory, tumor-suppressive (M1) state to an immunosuppressive, tumor-promoting (M2) state. In cancer, alternatively polarized macrophages secrete pro-tumorigenic cyto- and chemokines that suppress effector T cell activation and may prevent their access to the tumor microenvironment. In this study, we aim to determine if RON kinase inhibition can effectively modulate the pancreatic cancer microenvironment to promote antitumor activation states in macrophages.
Methods:
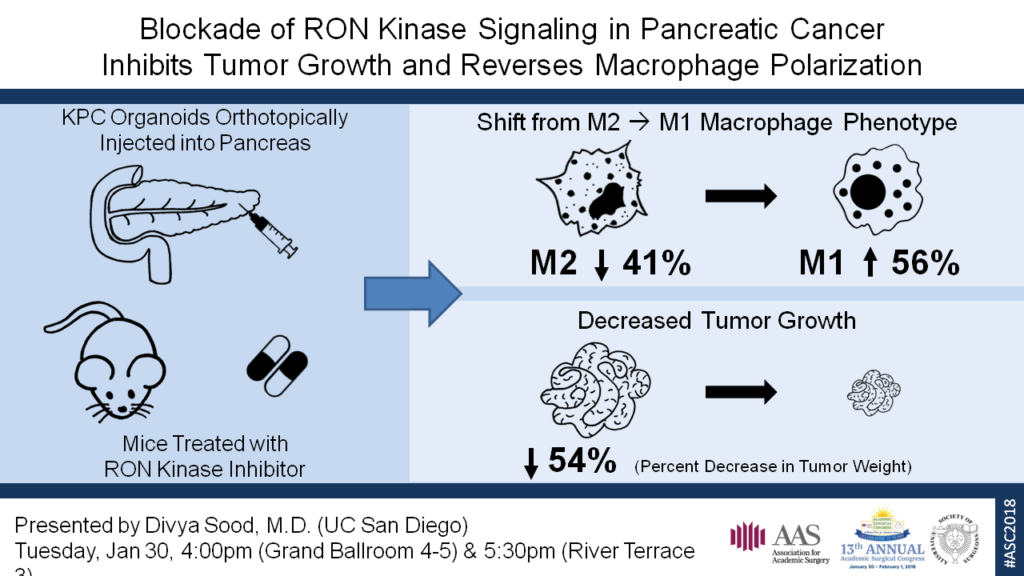
KPC (Pdx1-Cre; K-Ras+/LSLG12D; p53R172H/+) organoids were orthotopically injected into the pancreas of F1 hybrid B6-129S mice. LY2801653 is an orally bioavailable small molecule RON inhibitor in Phase II trials for advanced solid cancers. IPI549, a PI3Kγ inhibitor in Phase I trials, shown to have potent preclinical antitumor activity and promote classical activation of macrophages to the M1 state, was selected as a positive control. Treatment was started one week after injection for the following groups: A) vehicle control (10% acacia gum/5% NMP), B) LY2801653 12 mg/kg, C) LY2801653 24 mg/kg, D) IPI549 15 mg/kg, and E) LY2801653 24 mg/kg + IPI549 15 mg/kg. Mice were treated daily via orogavage for 2 weeks. The tumors were harvested and dissociated into a single cell suspension. The cells were labeled with fluorescent-conjugated antibodies to markers of leukocytes and macrophage polarization for flow cytometry. One way ANOVA with a Bonferroni correction for multiple comparisons was used to analyze the results.
Results:
Macrophages were defined as cells that labeled positive for CD45, CD11b, MHC II, and F4/80. Within that population, cells that labeled positive for CD80 and CD86, but negative for MRC1 were considered M1 macrophages, whereas those that were MRC1 positive, but negative for CD80 and CD86 were considered M2 macrophages. Flow cytometry demonstrated a significant decrease in the M2 macrophage population in each of the four treatment groups when compared to the vehicle control (p < 0.001). There was also a corresponding increase in the M1 macrophage population; this was significant (p < 0.001) for all except group E. Finally, mice in all four treatment groups exhibited significantly suppressed tumor growth by weight, relative to the vehicle control-treated mice (p < 0.005).
Conclusion:
In this study, we demonstrate that inhibition of RON kinase signaling alters the immunosuppressive microenvironment of pancreatic cancer and can be effectively targeted to shift that environment toward an antitumor state. Further studies are underway to better understand RON’s potential as a therapeutic target in pancreatic cancer.