A. K. Zamora1,2, D. Lascano1, H. Saven1, G. E. Asuelime1, J. Parmentier1, E. S. Kim1,2 1Children’s Hospital Los Angeles,Pediatric Surgery,Los Angeles, CA, USA 2University Of Southern California,Surgery,Los Angeles, CA, USA
Introduction:
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood with poor prognosis for children with high-risk disease. While nearly 80% of patients achieve initial remission, the majority succumb to recurrent, incurable metastatic disease. We have previously described our orthotopic murine model of minimal residual disease (MRD), which facilitates the in vivo development of metastatic disease, from which we derived site-specific metastatic cell lines. Metastatic neuroblastoma cells were analyzed by RNA-seq and demonstrated a marked upregulation of PHYHD1, a gene responsible for fatty acid oxidation and energy metabolism. Therefore, we hypothesize that metastatic NB cells differ in energy metabolism compared to primary tumor cells.
Methods:
Primary tumor, liver metastasis, and bone marrow metastasis cells derived from the human NB cell line NGP-fluc were incubated in normoxic (21% O2) and hypoxic (1% O2) conditions. After a 5-day incubation, oxidative phosphorylation and glycolysis rates were measured by a live-cell cellular metabolism assay (Agilent Seahorse XFp). In a separate experiment, a daily live-cell phase-count imaging proliferation assay was performed to assess individual cell line growth rates. Analysis of variance (ANOVA) and paired t-tests were performed. P<0.05 is significant
Results:
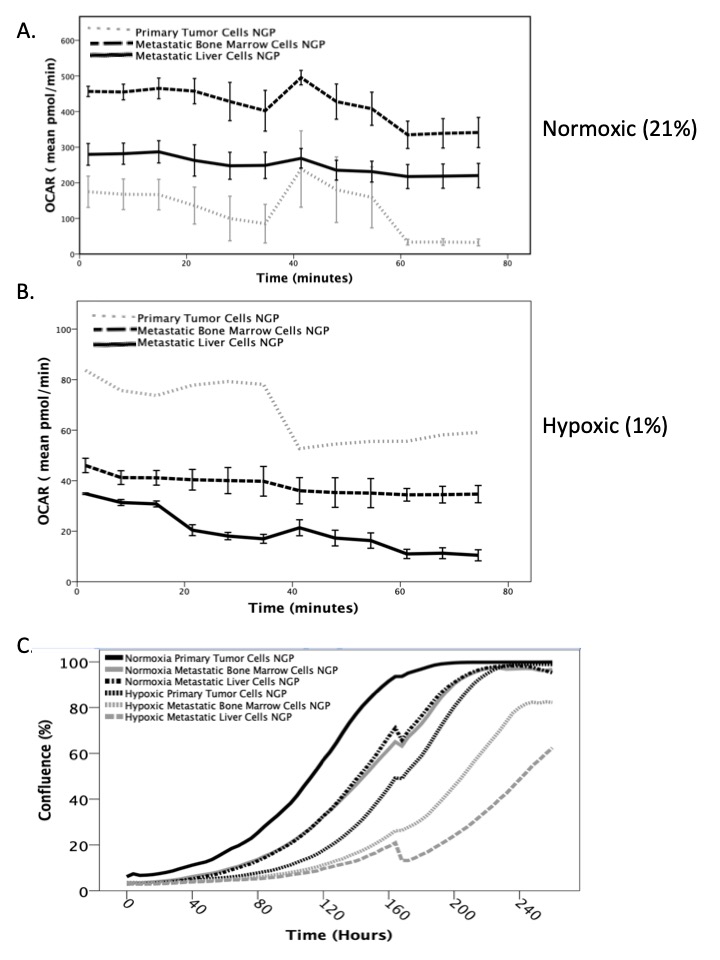
Metastatic liver and bone marrow neuroblastoma cells exhibited significantly increased oxidative phosphorylation (aerobic respiration) compared to primary tumor cells in normoxic conditions (p<0.005, Figure 1A). However, in hypoxic conditions, metastatic cells had significantly decreased oxidative phosphorylation and oxygen consumption (p<0.005, Figure 1B), which suggests decreased energy consumption. All NB cells have significantly lower basal metabolic rates under hypoxic conditions, as well as significantly slower proliferation rates than under normoxic conditions (Figure 1C, p< 0.005), with metastatic cells proliferating slower than the primary tumor cells (p< 0.005).
Conclusion:
In our study, metastatic NB cells exhibit significantly higher aerobic respiration under normoxia, but significantly lower cellular metabolism and proliferation under hypoxia than primary tumor cells. This cellular quiescence may explain how metastatic cells evade the immune system and subsequently recur. Additional in vitro and in vivo studies are ongoing to validate this phenomenon and to identify a point of intervention to inhibit metastatic disease.