C. M. Courtney1, K. M. Seiler1, E. J. Onufer1, J. Guo1, B. W. Warner1 1Washington University,Pediatric Surgery,St. Louis, MO, USA
Introduction: Short bowel syndrome resulting from massive small bowel resection (SBR) is one of the most lethal diseases of childhood and is associated with a perturbed gut microbiome and steatohepatitis. Our lab has previously shown increased bacterial translocation and intestinal paracellular permeability following SBR in a murine model. The purpose of this study was to test the hypothesis that altered intestinal permeability after SBR is regulated by toll-like receptor 4 (TLR4) – the main receptor for bacterial endotoxin.
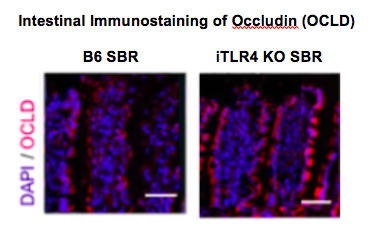
Methods: B6 mice and mice with intestinal specific TLR4 knock-out (iTLR4-KO) underwent 50% proximal SBR and were maintained on a liquid diet for 7 days. Paracellular permeability was evaluated in both groups using serum FITC-dextran absorption. Tight junctions (TJ) serve as the primary mediator of paracellular transport and were examined using electron microscopy (EM). In parallel experiments, intestinal epithelium was analyzed via single cell RNA sequencing to evaluate changes in expression of integral TJ protein, occludin (OCLD). Western blot technique and immunofluorescent microscopy were used to confirm the results of the single cell RNA sequencing experiments.
Results: FITC-dextran uptake into the serum was significantly decreased in iTLR4 KO mice compared to B6 mice following massive SBR (0.78 ± 0.31 ng/µL vs. 1.93 ± 0.29 ng/µL, p<0.01, n=8 per group). On electron microscopy, B6 SBR mice had distorted TJ structures while they were preserved in iTLR4 KO SBR mice. Single cell RNA sequencing revealed a 30% increase in OCLD in iTLR4 KO mice compared to B6 mice (p<0.05). Similarly, expression of OCLD was increased in iTLR4 KO mice compared to B6 mice on both immunostaining as well as Western blot analysis.
Conclusion: We have identified a pathway by which massive SBR impairs the gut barrier. Permeability is increased after SBR via changes in integral tight junction protein expression which appears to be regulated by TLR4. These findings suggest potential mechanistic targets for restoring the intestinal barrier function and obviating the adverse sequelae of short bowel syndrome.