A. Lam1, O. Zaborina1, J. Alverdy1 1University Of Chicago,Department Of Surgery,Chicago, IL, USA
Introduction:
Recent advancements in endoscopic surgery have spurred a rise in endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) for the treatment of early stage esophageal malignancy. Complications from EMR/ESD include stricture formation, full thickness injury, delayed perforation, and leak. Therefore a more complete understanding of the mechanisms that govern esophageal epithelial regeneration following injury is needed to prevent the occasionally lethal complications. Here, we describe a simple model for culturing whole esophageal explants long term, providing a platform for potentially studying regeneration ex vivo.
Methods:
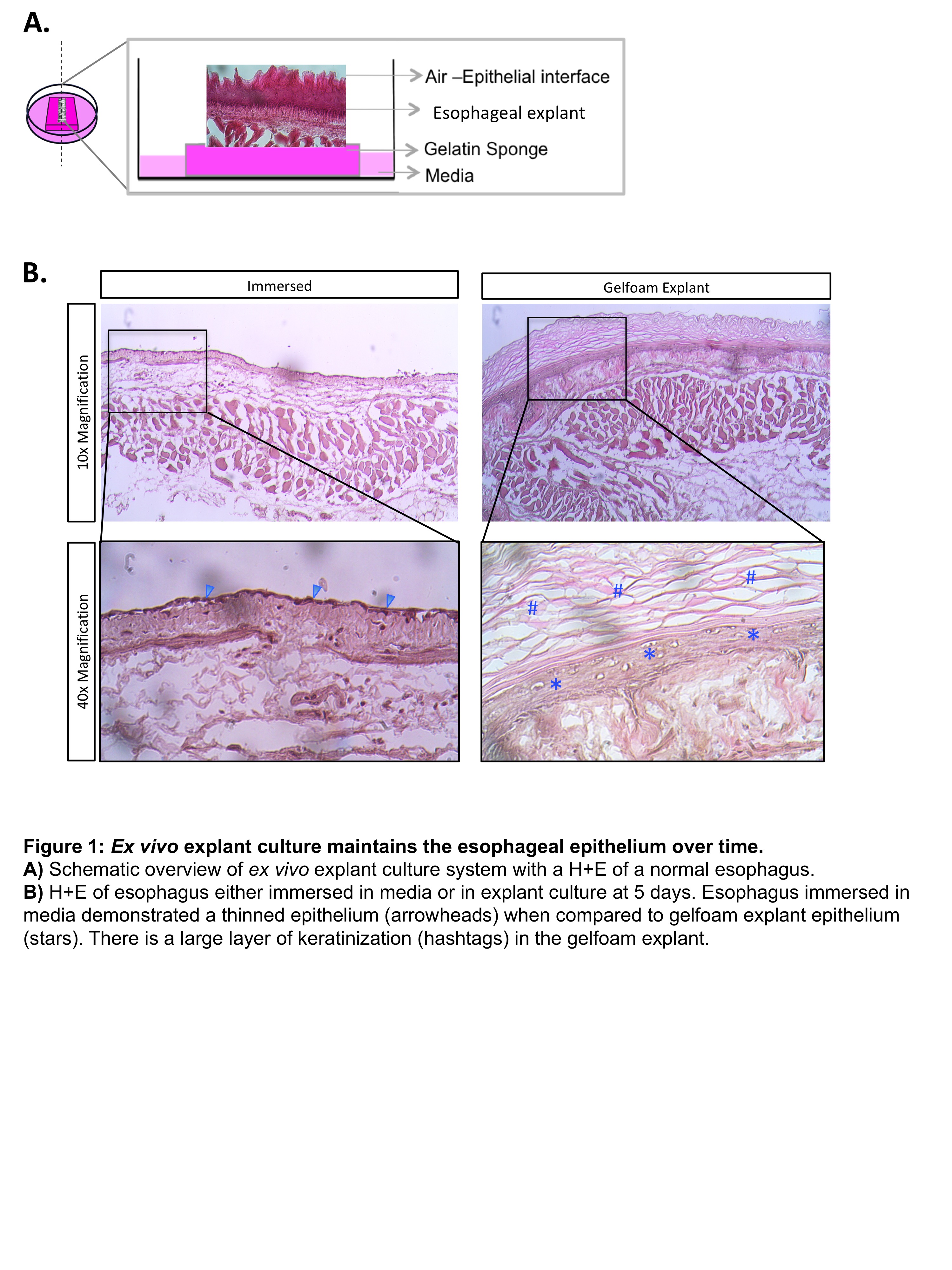
C57/BL6 wildtype mice were sacrificed and underwent sternotomy and laparotomy. The esophagus was dissected, opened longitudinally, and then either completely immersed in media or transferred atop a gelfoam sponge impregnated with media (DMEM/F12 with HEPES and Penicillin/Streptomycin). Following culture, tissues were fixed and cryosectioned in standard fashion. H&E staining was used to analyze explant architecture.
Results:
After the esophagus is completely immersed in culture for 5 days, there is loss of normal esophageal architecture characterized by atrophy of the esophageal mucosa. In contrast, when the esophagus is cultured atop a gelfoam sponge (mimicking an air-liquid interface), mucosal architecture is maintained. Furthermore, these explants also showed signs of mucosal proliferation, as evidence by an enlarged zone of keratinization. Additional studies demonstrated that this novel culture platform was able to maintain esophageal explants for up to 17 days without significant distortion of architecture.
Conclusion:
Long term whole organ esophageal explants can be maintained without compromising tissue integrity. This platform provides a novel yet simple method for potentially studying the process of esophageal regeneration following both superficial (i.e EMR) and deep (ESD) simulated injury in the mouse.