C. Fiechter1, 2, K. Scheurlen1, A. Littlefield1, T. Alfieri1, S. Galandiuk1 1Price Institute of Surgical Research, Division Of Colorectal Surgery, Hiram C. Polk Jr. Department Of Surgery, University Of Louisville School Of Medicine, Louisville, KY, USA 2University Of Louisville, School Of Medicine, Louisville, KY, USA
Introduction: The incidence of early-onset colorectal cancer (EOCRC) (<50 years of age) has been steadily rising, while the prevalence of obesity is increasing among developed countries. Inflammation is a central pathophysiological mechanism in both CRC and obesity. Tumor-associated macrophages (TAMs) and the obesity-related hormones leptin and adiponectin mediate inflammation and affect tumor progression in CRC. Current evidence shows that anti-inflammatory M2-like TAMs are associated with tumor progression and worse patient prognosis in CRC. The macrophage-specific metabolite itaconate is produced by the enzyme Aconitate Decarboxylase 1 (ACOD1) in certain macrophage subtypes and is carcinogenic. The effects of obesity-related hormones and itaconate on cellular metabolism in CRC, however, are largely unknown. The aim of this study was to investigate inflammatory responses and the role of itaconate in TAMs and colon cancer cells using an in vitro co-culture model.
Methods: THP-1 monocyte cells were seeded into 24-well co-culture plates and polarized into M2-like macrophages within 14 days. Cells were then co-cultured with HT-29 colon adenocarcinoma cells and treated with either leptin, adiponectin, 4-octyl itaconate (OI) or dimethyl itaconate (DI) for 3 and 6 hours. Gene expression was analyzed using TaqMan qRT-PCR. Statistical analysis was performed using a Student’s t-test.
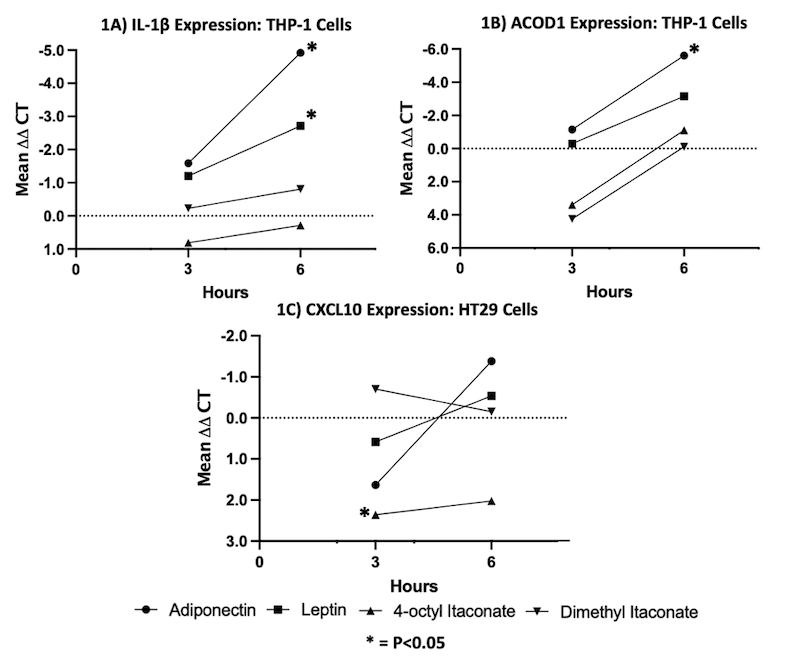
Results: In M2-like macrophages, proinflammatory IL-1β expression was significantly upregulated following adiponectin (30-fold, p=0.014) and leptin treatment (6-fold, p=0.026) for 6 hours (Fig. 1A). Expression of ACOD1 in M2-like macrophages was upregulated with adiponectin treatment (50-fold, p=0.002) for 6 hours (Fig. 1B). In HT-29 cells, OI treatment resulted in decreased expression of the proinflammatory cytokine CXCL10 at 3 hours (-5 fold-regulation, p=0.045) (Fig. 1C).
Conclusion: Adiponectin and leptin treatment results in a proinflammatory cellular response in TAMs, while the production of the carcinogenic and anti-inflammatory metabolite itaconate is induced. Therefore, the obesity-related hormones leptin and adiponectin activate tumor promoting mechanisms in CRC. Furthermore, the itaconate derivative OI decreases proinflammatory CXCL10 expression in colon cancer cells, thereby likely enhancing mechanisms of tumor growth and metastasis. In summary, the effects of adiponectin and leptin on M2-like TAM metabolism and cytokine expression in colon cancer may provide a link between obesity, inflammation and EOCRC.