A. Biswas1, H. J. Leraas2, S. Zadey2, P. Wilson3, B. Theriot4, N. Surana4, R. Ssekitoleko5, J. Mugaga5, C. Salzman1, A. Hall6, A. Saterbak1, T. Fitzgerald2 1Duke University, Biomedical Engineering, Durham, NC, USA 2Duke University, Surgery, Durham, NC, USA 3Duke University, Mechanical Engineering, Durham, NC, USA 4Duke University, Pediatrics, Durham, NC, USA 5Makerere University, Health Sciences, Kampala, Uganda 6Duke University, Pathology, Durham, NC, USA
Introduction: Gastroschisis mortality in sub-Saharan Africa remains high at 59-100%. Silo inaccessibility contributes to this extreme disparity, as standard of care (SOC) silos cost $280, and most median family incomes in sub-Saharan Africa are <$200 per month. Our team of surgeons, engineers, and neonatologists from the U.S. and Uganda have previously described design and bench-testing of a low-cost (LC) silo that costs <$5 and is constructed from a urine collection bag and female condom ring, which are locally available in sub-Saharan Africa. Here we describe in vivo testing of the LC silos.
Methods: A piglet model of gastroschisis was achieved by eviscerating the intestines through a 2cm midline abdominal wall incision. The bowel was placed into either a LC or SOC silo, maintained for one hour and then reduced. Procedure times for placement, intestinal reduction and silo removal were recorded. Tissue injury was assessed by protocol-blinded histological examination of the abdominal wall and intestine. Quantitative assessment of bacterial and fungal growth on the silos were performed using standard culture techniques. Data were analyzed using the Mann-Whitney test (alpha=0.05).
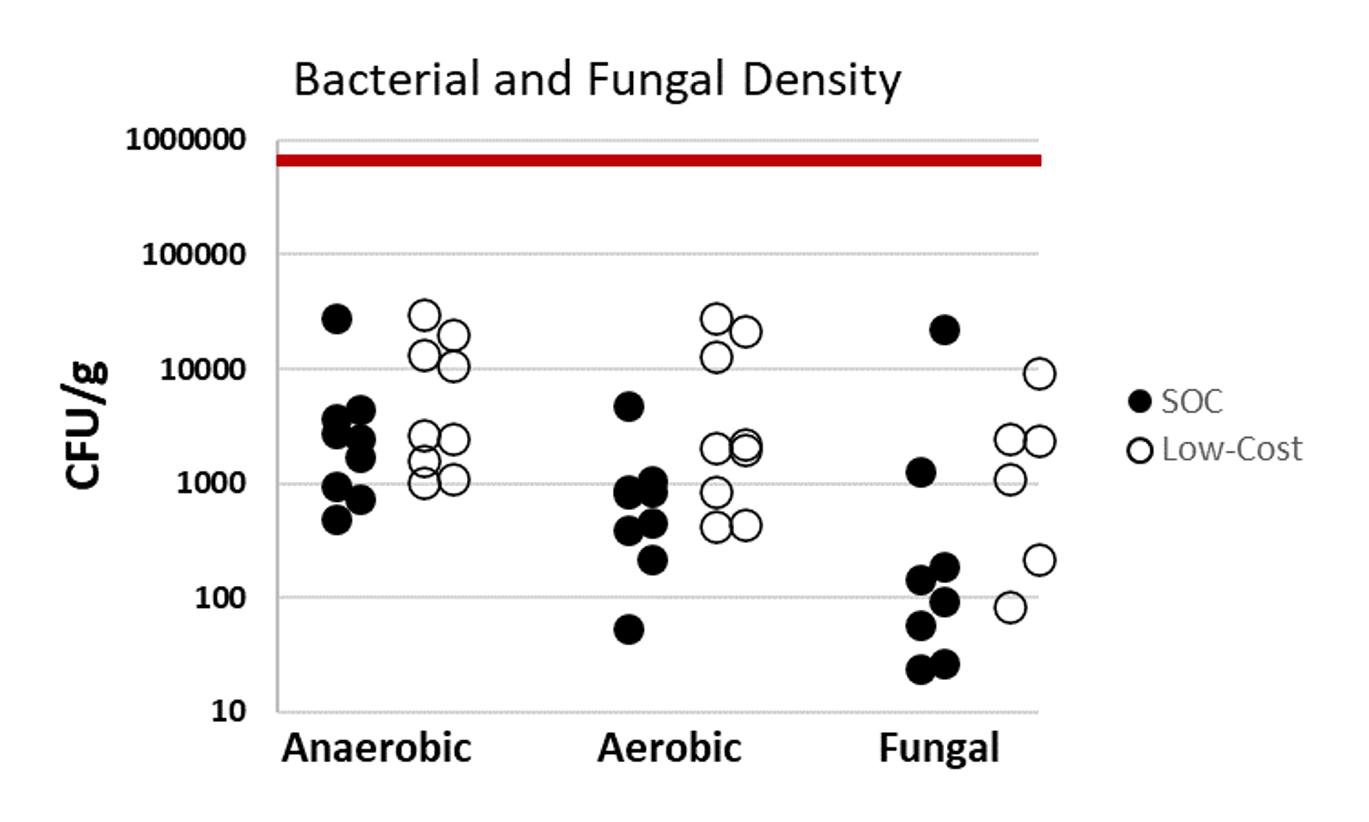
Results: Six piglets (2.0-2.6 Kg) were block randomized to LC (n=3) or SOC (n=3) silo application. There was no visible gross injury to abdominal wall or intestine specimens in either the LC or SOC silos. Concurrently, there was no difference in minor bleeding (bruising) between the LC and SOC silos (Table 1). The differences in silo application times (LC 42 ± 48 seconds; SOC 47 ± 18 seconds; p=1.0), bowel reduction times (LC 6 ± 3.5 seconds, SOC 1 ± 2 seconds; p=0.40) and silo removal times (LC 2 ± 0.5 seconds, SOC 3 ± 1.5 second; p=0.40) between groups were not statistically or clinically significant, indicating similar ease of use. Microbiology analysis revealed bacterial growth on all samples, but the bacterial density was below the standard inoculum in vitro acceptability of 105 CFU/g for both the LC and SOC silos (Fig 1). There was no statistically significant difference in bacterial or fungal growth between the LC and SOC silos (anaerobic p=0.34; aerobic p=0.06; fungal p=0.80).
Conclusion: A low-cost silo, designed for manufacturing and clinical use in sub-Saharan Africa, demonstrated similar ease of use, absence of tissue injury, and acceptable microbiology profile similar to SOC silos. Data from this porcine model will allow our team to proceed with a pilot clinical study in Uganda.