A. Labora1, H. Lee2, K. Rashid2, T. Le2, C. Chan1, T. Yamao1, E. Abt2, J. Link1, A. Premji1, A. Creech2, L. Li1, N. Wu1, C. Radu2, T. Donahue1 1University Of California – Los Angeles, Surgery, Los Angeles, CA, USA 2University Of California – Los Angeles, Pharmacology, Los Angeles, CA, USA
Introduction: The lack of biologically relevant preclinical models constitutes a major barrier to improving therapies for pancreatic cancer. Although most patients with pancreatic cancer present with metastatic disease, preclinical studies disproportionately rely on subcutaneous and orthotopic models. Previously described liver metastatic models such as the portal vein and intrasplenic injection methods employ open surgical procedures that are time-consuming and invasive. We report a novel technique to generate liver metastases via an ultrasound-guided splenic vein injection.
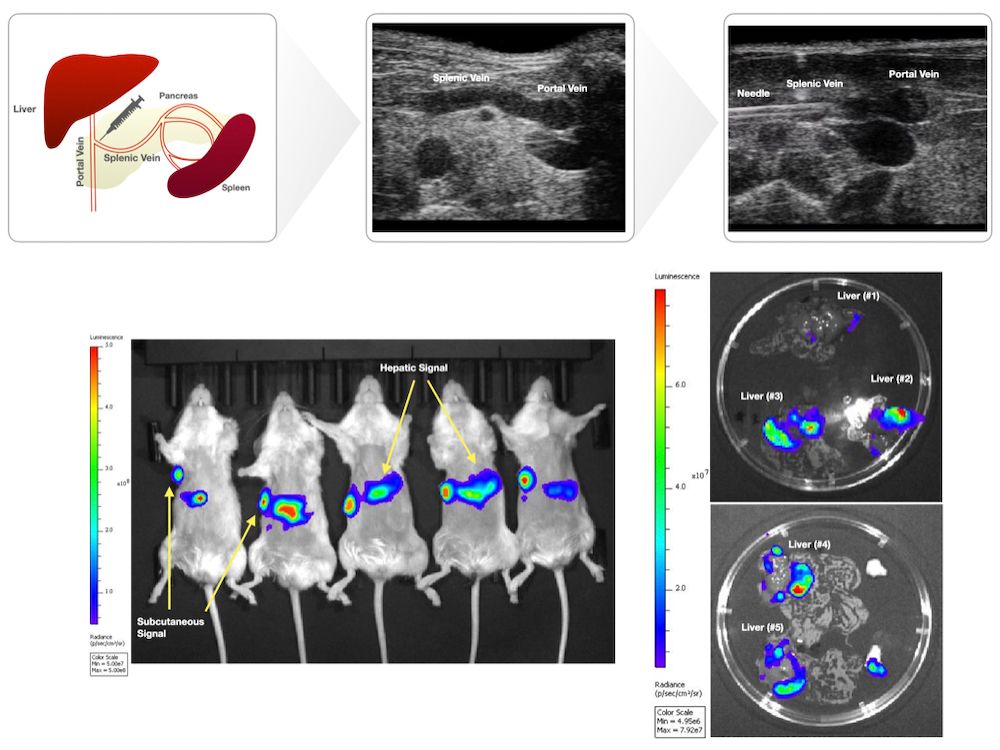
Methods: Ultrasound-guided splenic vein injection: Prior to injection, mice underwent hair removal. Mice were fasted for 12 hours prior to injection. Anesthetized mice were secured in the supine position on the pre-heated imaging platform. Ultrasound gel was applied, and the linear transducer (Vevo 2100) was used to locate anatomical landmarks (left kidney, stomach, spleen). A safe injection window whereby the splenic vein could be accessed at its confluence with the portal vein was established. Probe pressure was applied as needed to displace overlying viscera. The needle was positioned in-plane and advanced using toothed forceps to provide counter tension. The needle was advanced through the pancreas to access the splenic vein. Intravascular placement was confirmed by aspirating blood. Cells were subsequently injected into the vein. Following injection, the needle was slowly retracted under ultrasound guidance. Probe pressure was applied for several minutes, and the area was observed to ensure that no trauma had occurred. The mice were observed post-procedure to ensure adequate recovery. Animals: Female NSG mice, aged 8-to-10 weeks (N = 5). Cell Culture and Injection Preparation: HPAC cells expressing luciferase (HPAC-luc) were maintained in DMEM/F12 (1:1) with 10% FBS at 37°C in 5% CO2. HPAC-luc cells (1×106 cells) were trypsinized, washed thrice in PBS, and resuspended in PBS (20 μL for splenic vein injections, 100 μL for subcutaneous injections) prior to being loaded in 1 mL luer lock syringes with 30 G needles for splenic vein injections; 29.5 G insulin syringes were used for subcutaneous injections.
Bioluminescent imaging: Mice were injected intraperitoneally with 50 mg/kg D-Luciferin and imaged with the IVIS Lumina III imaging system. Data were analyzed using Living Image v4.5 software.
Results: Mice were imaged by BLI 22 days post-procedure and were found to have detectable BLI signal at both the subcutaneous site and hepatic region. Ex vivo BLI was performed on excised livers to confirm hepatic signal.
Conclusion: Injection into the splenic vein under ultrasound guidance is feasible and consistently generates liver metastasis.