N. H. Goldhaber1, T. Dong2,4, A. T. Phung4, J. R. Shah4, C. Larson3, A. B. Sanchez2, O. Aisagbonhi5, B. Oronsky2, W. C. Trogler4, T. Reid3, A. C. Kummel4, S. L. Blair1 1University Of California – San Diego, Department Of Surgery, San Diego, CA, USA 2University Of California – San Diego, EpcientRx Inc, San Diego, CA, USA 3University Of California – San Diego, Medical Oncology, San Diego, CA, USA 4University Of California – San Diego, Chemistry And Biochemistry, San Diego, CA, USA 5University Of California – San Diego, Department Of Pathology, San Diego, CA, USA
Introduction:
Oncolytic viruses can selectively infect tumor cells potentially inducing selective tumor cell lysis and activation of an immune response that has the potential to turn a “cold tumor” (one not attacked by the immune system) into a “hot tumor” (one susceptible to attack by the immune system) by the release of immune-stimulatory cytokines and molecules. Turning an immunosuppressed “cold tumor” into a “hot tumor” by increasing immune cells inside an oncolytic virus infected tumor is a key to improving anticancer activity.
Adenoviruses (Ad) as an oncolytic viral therapy has shown therapeutic effects on local treatment of cancers in clinical trials. However, its more widespread use is hindered not only by the requirement of the tumor cells to have a coxsackievirus and adenovirus receptor (CAR) for effective infections but also by the requirement for local administration (injection into a tumor).
Methods:
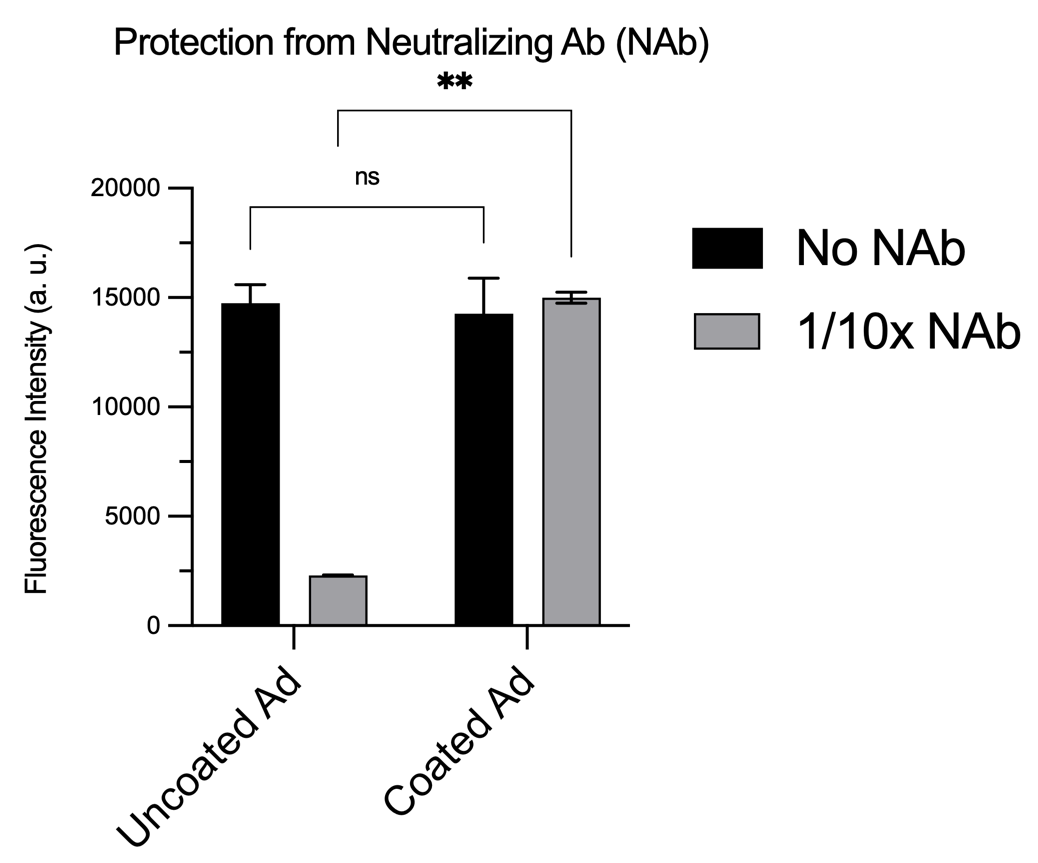
A liposome encapsulated adenovirus platform was developed to efficiently infect CAR-deficient cancer cells. The encapsulation of Ad by using cationic liposomes is the key to overcoming CAR-dependent infections and generating a strong immune response against infected tumor cells. The liposome encapsulation also provided a shield to allow Ad to escape from neutralizing antibodies (NAb) that may already exist in the blood. 90 nm diameter adenoviruses were encapsulated in 200nm extruded DOTAP-folate liposomes (Df) by simple incubation. The encapsulation process relies on simple charge interactions, and these encapsulated Ads (DfA) are able to infect CAR deficient tumor cells.
Results:
The encapsulated Ads (DfA) were tested in vitro first by incubating with neutralizing antibodies (NAb[BS1] ), and the results showed protection against the 1/10x neutralizing serum[BS2] (p value<0.005) which may further improve the efficacy of multiple doses of treatment.
The damage-associated molecular patterns (DAMPs), including extracellular ATP, HMGB-1 and CRT, confer an adjuvanticity to dying cancer cells, as they facilitate the recruitment and activation of immune cells, including APC, T cells and NK cells. The significant release of immunogenic molecules by DfA was assessed by transfecting CAR-deficient cells in vitro. There was significant more release of extracellular ATP (3200 vs 500 relative luminescence, p<0.05) and HMGB-1 (12 vs 3 ug/L, p<0.05).
Conclusion:
In conclusion, a DOTAP-folate encapsulated adenovirus has been shown to infect, lyse and induce a robust systemic immune response against CAR-deficient cancer cells. In the future, this construct can be utilized to improve efficacy of tumor vaccines strategies to prevent tumor recurrence.