A. K. Zamora1,2, D. Lascano1, H. Saven1, G. E. Asuelime1, J. Parmentier1, E. S. Kim1,2 1Children’s Hospital Los Angeles,Pediatric Surgery,Los Angeles, CA, USA 2University Of Southern California,Surgery,Los Angeles, CA, USA
Introduction:
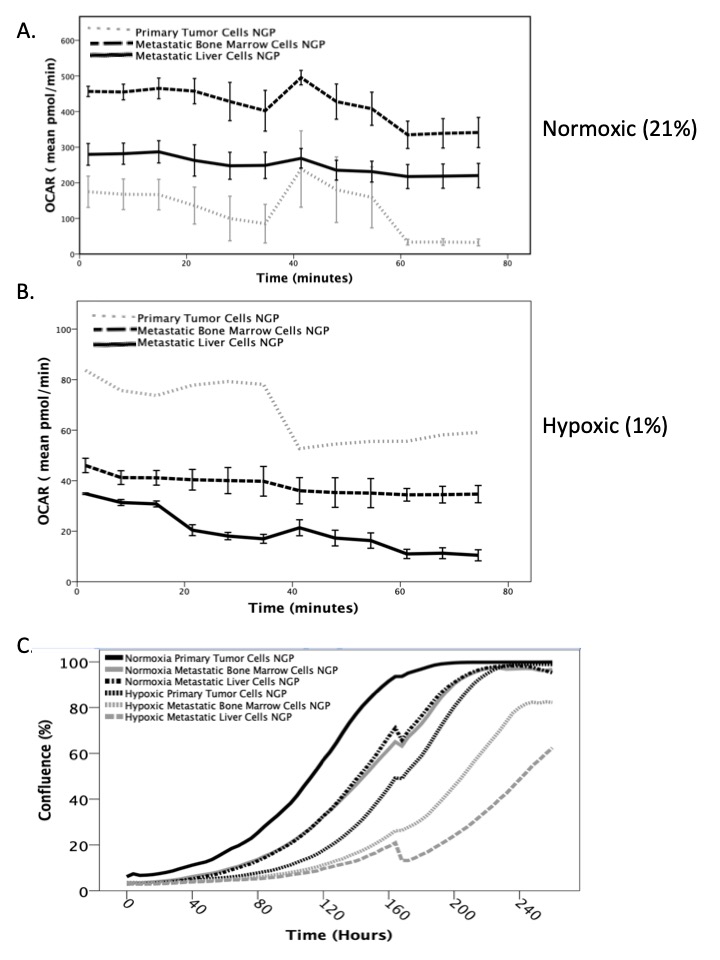
Neuroblastoma (NB) is the most common extracranial solid tumor of childhood with poor prognosis for children with high-risk disease. While nearly 80% of patients achieve initial remission, the majority succumb to recurrent, incurable metastatic disease. We have previously described our orthotopic murine model of minimal residual disease (MRD), which facilitates the in vivo development of metastatic disease, from which we derived site-specific metastatic cell lines. Metastatic neuroblastoma cells were analyzed by RNA-seq and demonstrated a marked upregulation of PHYHD1, a gene responsible for fatty acid oxidation and energy metabolism. Therefore, we hypothesize that metastatic NB cells differ in energy metabolism compared to primary tumor cells.
Methods:
Primary tumor, liver metastasis, and bone marrow metastasis cells derived from the human NB cell line NGP-fluc were incubated in normoxic (21% O2) and hypoxic (1% O2) conditions. After a 5-day incubation, oxidative phosphorylation and glycolysis rates were measured by a live-cell cellular metabolism assay (Agilent Seahorse XFp). In a separate experiment, a daily live-cell phase-count imaging proliferation assay was performed to assess individual cell line growth rates. Analysis of variance (ANOVA) and paired t-tests were performed. P<0.05 is significant
Results:
Metastatic liver and bone marrow neuroblastoma cells exhibited significantly increased oxidative phosphorylation (aerobic respiration) compared to primary tumor cells in normoxic conditions (p<0.005, Figure 1A). However, in hypoxic conditions, metastatic cells had significantly decreased oxidative phosphorylation and oxygen consumption (p<0.005, Figure 1B), which suggests decreased energy consumption. All NB cells have significantly lower basal metabolic rates under hypoxic conditions, as well as significantly slower proliferation rates than under normoxic conditions (Figure 1C, p< 0.005), with metastatic cells proliferating slower than the primary tumor cells (p< 0.005).
Conclusion:
In our study, metastatic NB cells exhibit significantly higher aerobic respiration under normoxia, but significantly lower cellular metabolism and proliferation under hypoxia than primary tumor cells. This cellular quiescence may explain how metastatic cells evade the immune system and subsequently recur. Additional in vitro and in vivo studies are ongoing to validate this phenomenon and to identify a point of intervention to inhibit metastatic disease.
L. Bownes1, A. Williams1, R. Marayati1, C. Quinn1, L. Stafman1, J. Stewart1, P. Datta2, J. Easlick3, E. Beierle1 1University Of Alabama at Birmingham,Division Of Pediatric Surgery,Birmingham, AL, USA 2University Of Alabama at Birmingham,Division Of Hematology/Oncology,Birmingham, AL, USA 3University Of Alabama at Birmingham,Division Of Transplantation,Birmingham, AL, USA
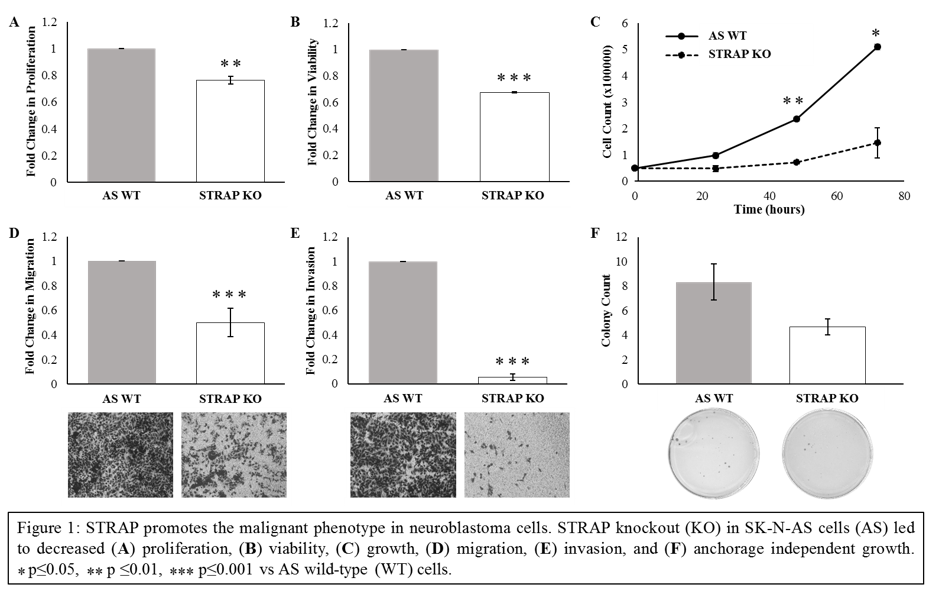
Introduction: Serine-threonine kinase receptor associated protein (STRAP) is upregulated in several malignancies and plays an important role in tumor growth and metastasis. The role of STRAP in pediatric malignancies and specifically in neuroblastoma (NB) has not been explored. We sought to determine whether STRAP functions to promote the malignant phenotype in NB.
Methods: CRISPR-Cas9 gene editing was utilized to establish stable genetic knockout (KO) of Strap in the human NB cell line SK-N-AS (AS). Immunoblotting confirmed STRAP KO. Growth curves for STRAP wild type (WT) and KO cells were documented at 24, 48, and 72 hours and cell survival and proliferation were compared using alamarBlue and CellTiter96 assays, respectively. Differences in cell motility were assessed using modified Boyden chamber assays. Anchorage independent growth was evaluated using soft agar assay. To investigate the role of STRAP in NB tumorigenesis in vivo, 1.8×106 WT or KO cells were injected into flanks of 6-week old athymic nude mice. Tumor volumes were measured with calipers three times per week. Splenic injections of WT and KO cells followed by splenectomy were performed to explore an in vivo model of NB metastasis cells. Liver ultrasound was used to quantify liver metastasis.
Results: Cell proliferation and survival were significantly decreased in the KO cells by 23% (p=0.002) and 32% (p=0.0001), respectively (Figure 1 A, B). Cell growth was also significantly decreased in the KO cells at 48 (p=0.004) and 72 (p=0.02) hours (Figure 1 C). STRAP KO cells showed significantly decreased migration (p<0.0001, Figure 1 D) and invasion (p<0.0001, Figure 1 E) compared to WT cells, and formed fewer colonies in anchorage independent growth conditions (p=0.09, Figure 1 F). In vivo, there was a decrease in average tumor volumes as well as relative tumor growth between mice injected with WT and KO cells. The metastatic model also demonstrated a trend toward decreased number (5 vs. 8) and average volume (26.7 vs. 65.4 cm3, p=0.1) of liver metastases in mice injected with KO compared to WT cells.
Conclusion: STRAP KO in NB cells led to decreased cell growth, survival, proliferation, and motility in vitro, as well as a trend toward decreased tumor volume and metastatic burden in vivo. These novel findings demonstrated that STRAP plays a role in promoting the malignant phenotype in NB and warrants further investigation as a potential therapeutic target in NB.
C. M. Courtney1, K. M. Seiler1, E. J. Onufer1, J. Guo1, B. W. Warner1 1Washington University,Pediatric Surgery,St. Louis, MO, USA
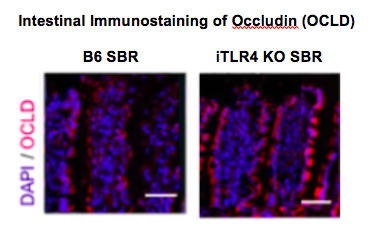
Introduction: Short bowel syndrome resulting from massive small bowel resection (SBR) is one of the most lethal diseases of childhood and is associated with a perturbed gut microbiome and steatohepatitis. Our lab has previously shown increased bacterial translocation and intestinal paracellular permeability following SBR in a murine model. The purpose of this study was to test the hypothesis that altered intestinal permeability after SBR is regulated by toll-like receptor 4 (TLR4) – the main receptor for bacterial endotoxin.
Methods: B6 mice and mice with intestinal specific TLR4 knock-out (iTLR4-KO) underwent 50% proximal SBR and were maintained on a liquid diet for 7 days. Paracellular permeability was evaluated in both groups using serum FITC-dextran absorption. Tight junctions (TJ) serve as the primary mediator of paracellular transport and were examined using electron microscopy (EM). In parallel experiments, intestinal epithelium was analyzed via single cell RNA sequencing to evaluate changes in expression of integral TJ protein, occludin (OCLD). Western blot technique and immunofluorescent microscopy were used to confirm the results of the single cell RNA sequencing experiments.
Results: FITC-dextran uptake into the serum was significantly decreased in iTLR4 KO mice compared to B6 mice following massive SBR (0.78 ± 0.31 ng/µL vs. 1.93 ± 0.29 ng/µL, p<0.01, n=8 per group). On electron microscopy, B6 SBR mice had distorted TJ structures while they were preserved in iTLR4 KO SBR mice. Single cell RNA sequencing revealed a 30% increase in OCLD in iTLR4 KO mice compared to B6 mice (p<0.05). Similarly, expression of OCLD was increased in iTLR4 KO mice compared to B6 mice on both immunostaining as well as Western blot analysis.
Conclusion: We have identified a pathway by which massive SBR impairs the gut barrier. Permeability is increased after SBR via changes in integral tight junction protein expression which appears to be regulated by TLR4. These findings suggest potential mechanistic targets for restoring the intestinal barrier function and obviating the adverse sequelae of short bowel syndrome.
M. T. Grant1, J. D. Vrecenak1 1Washington University,Pediatric Surgery,St. Louis, MO, USA
Introduction: In utero hematopoietic cell transplantation (IUHCT) is performed before the fetal immune system matures and thus requires neither myeloablation nor toxic immunosuppression, while allowing stable long-term engraftment of donor cells in a mixed chimeric state. Our laboratory has demonstrated that the fetal blood brain barrier is sufficiently permeable to allow for cellular trafficking of transplanted cells into the central nervous system (CNS) at a wide range of gestational timepoints in a murine model. Given the presence of donor-derived cells in this selective environment, we sought to investigate whether these cells were present in other highly regulated developmental niches such as the gonads. Furthermore, we sought to investigate whether mating IUHCT-derived chimeras would yield any detectable chimerism in offspring.
Methods: In preparation for IUHCT, BALB/c male mice were singly housed and paired with BALB/c females for a 24-hour period in order to achieve time-dated mating. Donor bone marrow (BM) was harvested from B6-GFP. Following ficoll separation, donor BM mononuclear cells were prepared for same-day injection. At gestational time points of 13 and 14 days, BALB/c dams found to be pregnant on exam underwent laparotomy and fetal intravenous injection of 20 x 106 GFP+ donor BM mononuclear cells per fetus via the vitelline vein. Resultant pups underwent peripheral blood chimerism analysis monthly through six months. Two male and two female pups found to have chimerism >20% were mated with untreated BALB/c mice of the opposite sex. The resultant offspring from those litters underwent chimerism analysis by flow cytometry at one month of age. Additionally, the first-generation chimeras underwent fluorescent stereomicroscopy of gonadal tissue at time of terminal harvest.
Results: As a result of IUHCT, 83% of the treated pups had chimerism levels greater than 20%. Both male and female chimeras yielded offspring of both sexes, none of which had any degree of chimerism in peripheral blood or bone marrow. Fluorescent stereomicroscopic examination of gonadal tissue from chimeras revealed low levels of fluorescence consistent with serosal engraftment, but no appreciable amount of fluorescence was present within the gonadal parenchyma.
Conclusion: Although donor-derived cells can engraft within tightly regulated developmental niches such as the CNS following IUHCT, the donor cells do not engraft within or alter the germline. Thus, the progeny of fetuses treated with IUHCT are not chimeric. This has significant clinical implications regarding reproductive planning for future patients treated with IUHCT, as their offspring could be affected by and/or carry the same genetic condition that necessitated treatment with IUHCT.
E. Mahdi1, A. Glazier1, N. Malkoff1, J. Xu1, N. Noriega1, C. Short1, M. Fenlon1, K. Wang1 1Children’s Hospital Los Angeles,Pediatric Surgery,Los Angeles, CA, USA
Introduction: Biliary atresia (BA), a congenital obstructive cholangiopathy, is the leading cause of end-stage liver failure in kids. We previously showed expansion of Prominin-1 (Prom1)-expressing hepatic progenitor cells (HPCs) within areas of periportal fibrosis in BA, with Prom1 knockout leading to decreased biliary ductular reactions and fibrosis typical of BA. Herein, we hypothesized that Prom1 functionally promotes fibrosis.
Methods: PROM1-enriched Mat1a-/- HPCs, which were treated in vitro ± 5 ng/mL profibrogenic recombinant (r) TGFB ± 0.05 µmol X030 (loss-of-function), which inactivates HSP90 downstream of PROM1, to assess effect on fibrogenic gene expression. A stably transfected Prom1-overexpressing Mat1a HPC clone (gain-of function) was then treated with ± 5 ng/mL rTGFB. Relative gene expression was analyzed using quantitative polymerase chain reaction. PROM1-positive and negative cells were collected separately by fluorescence-activated cell sorting (FACS) to assess relative gene fibrogenic expression.
Results: Following rTGFB treatment, Mat1a cells exhibited increased fibrogenic markers Collagen-1a (Col1α) (43.2±5.7, p<0.0001), a-Smooth muscle actin (aSma) (166.1±20.1, p<0.0001), Integrin-B6 (ItgB6) (4.4±0.4, p<0.0001), and Vimentin (2.3±0.7); increased progenitor markers Sox9 (2.8±0.2, p<0.0001) and Cd49f (1.9±0.2, p<0.0008); and decreased cholangiocyte marker Cytokeratin-19 (Ck19) (0.8±0.08, p<0.0116) expression. X030 co-treatment resulted in decreased Col1a (17.1±2.4, p<0.0001), ItgB6 (2.8±0.1, p<0.0001), Sox9 (4.0±0.1, p<0.0001), Cd49f (4.2±0.2, p<0.0001), and Ck19 (0.70±0.01, p<0.0001), but no change in aSma or Vimentin. Prom1-overexpressing cells exhibited a 13,996 (±1263, p<0.0003) fold increase in Prom1 compared to Mat1a cells. They showed no difference in baseline Col1a, aSMA, Vimentin, CK19, or Sox9 levels, but had decreased TGB6 (0.33±0.04, p<0.0001) expression. Prom1 over-expressed cells treated with TGFB, demonstrated increased Col1a (28.0±9.3, p<0.0006), aSma (35.8±3.7, p<0.0001), Vimentin (1.6±0.2, p<0.027), as well as decreased CK19 (0.8±0.1, p<0.04) compared to control. X030 co-treatment resulted in decreased Col1a (12.1±2.7, p<0.01) and aSma (8.4±2.6, p<0.0001), increased albumin (2.7±0.6, p<0.0197), and no change in Ck19 or Sox9. Compared to PROM1-negative cells, FACS-sorted PROM1-positive cells had no difference in Col1α and ITGB6, decreased CK19 (0.7±0.1, p<0.0001), and increased Vimentin (1.7±0.2, p<0.002) at baseline. After TGFB treatment, PROM1-positive cells had increased Col1α (880.3±230.2, p<0.0001) compared to PROM1 negative cells.
Conclusion: Prominin-1 functionally promotes a profibrogenic response in Mat1a null HPC in vitro.
A. K. Zamora1, M. J. Zobel1, J. Sun2, T. Lin2, M. Sheard2, R. C. Seeger2, E. S. Kim1 1Children’s Hospital Los Angeles,Pediatric Surgery,Los Angeles, CA, USA 2Children’s Hospital Los Angeles,Hematology Oncology,Los Angeles, CA, USA
Introduction:
Children with high-risk neuroblastoma (NB) have a poor prognosis with an overall survival of less than 50%. The anti-GD2 monoclonal antibody, dinutuximab, has been shown to improve survival but many children recur with resistant disease. We recently discovered that treating neuroblastoma cells with activated natural killer cells (aNK) and dinutuximab leads to tumor cell death and transfer of the neuroblastoma membrane marker GD2 to the natural killer cells, a phenomenon called trogocytosis. We characterize the process of trogocytosis following immunotherapy and hypothesize that these “experienced” NK cells have decreased efficacy in killing neuroblastoma cells.
Methods:
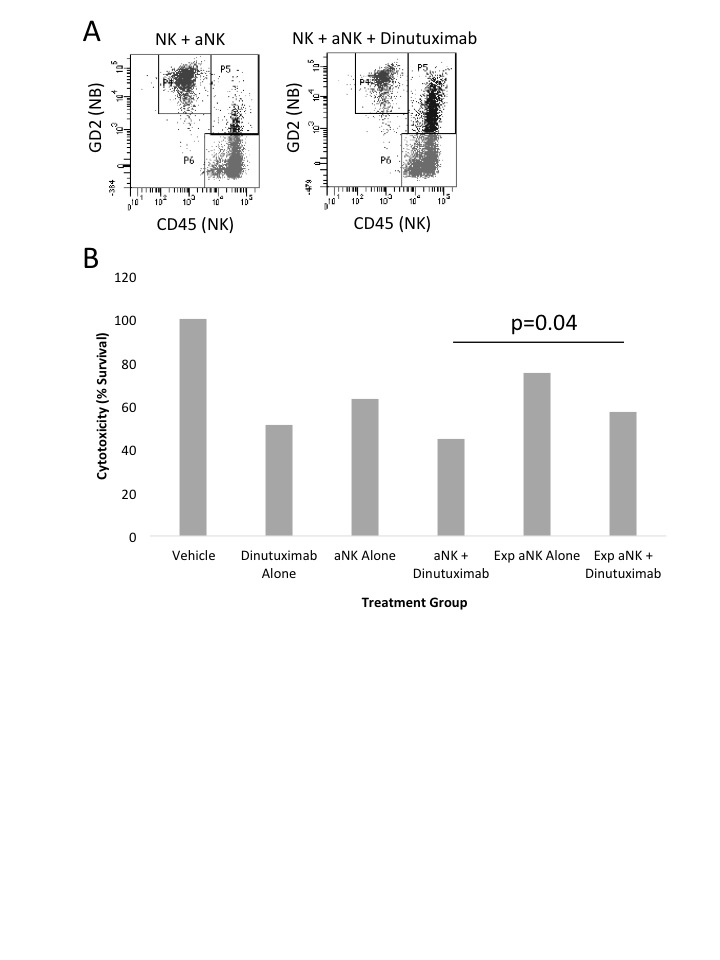
Human NB cells from the CHLA255-fluc cell line were treated with vehicle control, dinutuximab (100ng/mL) alone, aNK cells alone, or aNK cells plus dinutuximab. The treatment groups were then incubated, harvested hourly from 0 minutes to 6 hours, and analyzed by flow cytometry to evaluate GD2 (APC) and CD45 (AlexaFluor-488) expression. Live cell microscopy images were obtained at various timepoints. The “experienced” GD2+ NK cells were then incubated with fresh NB cells for 6 hours. Luciferase cytotoxicity assays were then performed to evaluate the efficacy of the “experienced” GD2+ NK cells compared to fresh NK cells. Student’s t-test was performed; p<0.05 is significant.
Results:
Utilizing flow cytometry, a novel GD2+/CD45+ population is observed and reaches maximal expression at 37.9% following aNK + dinutuximab treatment at 3 hours (Figure 1A). As tumor cells are killed, there is a notable time-dependent loss of GD2 expression (NB cells) with a concomitant rise in the GD2+/CD45+ cohort. Live cell imaging demonstrates the uptake of NB membrane-bound GD2 by the CD45+ NK cells, resulting in GD2+ NK cells. Lastly, the “experienced” GD2+ NK cells demonstrated significantly decreased cytotoxicity to NB cells compared to fresh NK cells (p=0.04, Figure 1B). This trogocytosis effect is dependent on dinutuximab as the cytotoxicity of fresh NK cells alone does not significantly differ from “experienced” NK cells alone (p=0.13).
Conclusion:
Our study demonstrates the novel phenomenon of trogocytosis, by which NK cells acquire the GD2 neuroblastoma membrane receptor following combination immunotherapy, which may represent a novel mechanism of resistance. Ongoing in vitro, in vivo, and patient specimen studies are being conducted to further characterize and validate the functional effects of trogocytosis on immunotherapy for neuroblastoma.
Y. Chun1, B. P. Soares2, W. Fulton1, C. Sodhi1, D. J. Hackam1, I. Nasr1 1The Johns Hopkins University School Of Medicine,Pediatric Surgery,Baltimore, MD, USA 2University Of Vermont College Of Medicine / Fletcher Allen Health Care,Radiology,Burlington, VT, USA
Introduction:
Traumatic brain injury (TBI) induces a robust neuroinflammatory response that activates the innate immune system. Signaling through the Toll-like receptor 4 (TLR4) pathway is one of the key mechanisms by which the innate immune system triggers the neuroinflammatory cascade in response to TBI. We hypothesize that the pharmacologic inhibition of TLR4 using the novel compound, C34 will lead to a reduction in the acute neuroinflammatory response and result in improved cognitive outcomes. We propose that the infiltrating monocytes play a role in attenuating the neuroimmune response.
Methods:
A murine controlled cortical impact TBI model was used. The experimental groups are: (1) wild-type (WT) control, (2) WT+C34, (3) CX3CR1GFP. CX3CR1 is a microglial marker. Cell sorting was used to isolate monocytes and microglial cells from the brain. Real-time PCR quantified cytokine gene expression. Morris Water Maze was used to assess cognitive outcomes. Cell sorting was used to separate microglia and monocytes from the brain parenchyma after TBI. Statistical analysis: Student’s T-test and One-way ANOVA.
Results:
Short-term TLR4 inhibition showed significant improvement in cognitive behavior. This was assessed using the Morris Water Maze which showed improved latency in week 1 and platform entries on week 4 (p=0.026). The treatment group showed a marked increase in infiltrating monocytes on post-injury day (PID) 7. CX3CR1 positive monocytes were identified in the spleen on PID 7 (n=5) but not on PID 1 (n=5). The cell sorting technique was used to phenotype the infiltrating monocytes as well as the resident microglia in the brain parenchyma. On PID 1, the infiltrating monocytes had significantly higher gene expression of the anti-inflammatory gene IL-10 (31.66±5.013, n=5) but decreased expression of the pro-inflammatory gene TNF? (512.5±59.35, n=5) compared to the expression in brain microglia: IL-10 (2.748±0.3728, n=5), and TNF? (1220±166.8, n=5). On PID 7, the infiltrating monocytes had higher gene expression of the anti-inflammatory gene IL-10 (7.068±1.79, n=4) but decreased expression of the pro-inflammatory genes IL-6 (8.898±0.7059, n=5) and TNF? (92.64±20.07, n=4) compared to the expression in brain microglia: IL-10 (1.604±0.4127, n=5), IL-6 (85.68±12.82, n=5), and TNF? (512.6±80.31, n=5).
Conclusion:
Our findings demonstrate that infiltrating monocytes play a key role in both the early and the delayed immune responses to TBI. Infiltrating monocytes displayed an anti-inflammatory gene expression profile in the injured brain on PID 1 and PID 7. These infiltrating monocytes express more anti-inflammatory cytokines compared to the resident microglial population. The increased number of these infiltrating anti-inflammatory monocytes potentially contributes to the improved cognitive outcomes seen in the C34 treated mice.
E. Dobrinskikh1, S. I. Al-Juboori2, U. Shabeka2, A. I. Marwan2 1University Of Colorado Denver,Medicine,Aurora, CO, USA 2University Of Colorado Denver,Division Of Pediatric Surgery, Department Of Surgery,Aurora, CO, USA
Introduction: The mammalian respiratory system is a highly specialized organ. The bidirectional interactions between endoderm and the surrounding mesoderm are important for coordinated differentiation of the respiratory epithelial cells, and the formation of vascular capillary network with a common basement membrane, which is crucial for gas exchange. Basement membrane components play a dynamic role as a barrier and reservoir for different growth factors. Proper expression and function of the basement membrane extracellular matrix (ECM) are necessary for normal lung growth and development. However, little is known how the proliferative state of the alveolar epithelial cells (AEC) can influence ECM composition. Previously we have shown that tracheal occlusion, an experimental therapeutic intervention used to induce lung growth, in normal lungs leads to the formation of at least two zones; with control-like signatures and zones with enlarged airspaces and a metabolic shift to glycolysis, which could indicate a presence of proliferating cells. Thus, in this study we tested the hypothesis that in zones with enlarged airspaces, type 2 AEC are shifted towards proliferation and therefore can lead to a change in the surrounding ECM composition.
Methods: RNAScope combined with immunofluorescence (IF), global proteomics, Fluorescence Lifetime Imaging Microscopy (FLIM), Second harmonic generation (SHG) imaging analyses were used to characterize control-like and enlarged airspaces in normal fetal rabbit lungs following 4 days of tracheal occlusion. Tissues were harvested at 30 days of gestation.
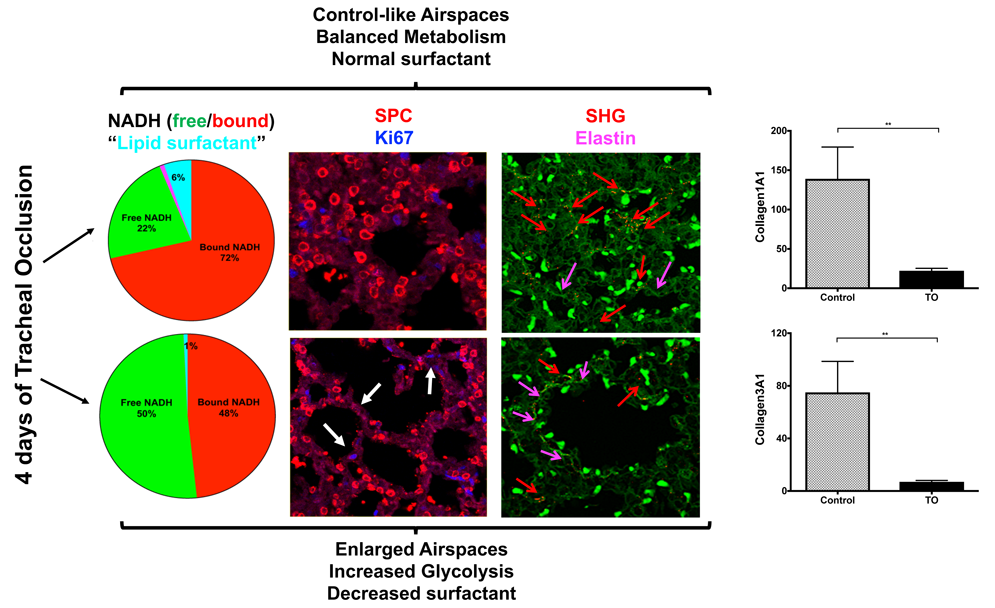
Results: RNAScope for SPC or SPA mRNA expression, in a combination with IF for proSPC protein and proliferation marker (Ki67), revealed almost absent double-positive AEC2 in control-like airspaces at this gestational age, whereas in the enlarged airspaces an increase in proliferating AEC2 was observed. Global proteomics of a whole lung lobe revealed a significant decrease in multiple ECM components; including collagen 4 (a major component of the basement membrane) in addition to collagens 1 and 3 (main structural collagens of different connective tissues, including parenchymal vasculature). FLIM showed that in the zones with a shift toward glycolysis and enlarged structures there was an appearance of a strong signal, corresponding to elastin lifetime. SHG confirmed increased signal for elastin in addition to a decrease of fibrillar collagens 1 and 3 nearby big airspaces.
Conclusion: For the first time we have shown that heterogeneous parenchymal changes following 4 days of tracheal occlusion are accompanied by changes in ECM components composition. These changes are correlated with increased number of proliferating AEC2 cells.
R. A. Clark1, S. Lee1, J. Qiao1, D. H. Chung1 1University Of Texas Southwestern Medical Center,Pediatric Surgery,Dallas, TX, USA
Introduction:
Pralatrexate is a folate analogue inhibitor of dihydrofolate reductase exhibiting high affinity for reduced folate carrier-1 (RFC-1) with antineoplastic and immunosuppressive activities that are similar to methotrexate. Pralatrexate is approved in the US for the treatment of relapsed or refractory peripheral T-cell lymphoma and is being investigated in various malignancies. Despite intense research advances, the overall survival for children with high-risk neuroblastoma has failed to improve. Therefore, the search continues for the best single agent or combination of chemotherapy drugs to treat these tumors with high relapse rate. Previous studies have demonstrated that the gene encoding RFC1, SLC19A1, is associated with MYCN amplification in neuroblastoma, suggesting a potential role for Pralatrexate. Herein, we sought to assess the inhibitory role of pralatrexate in neuroblastoma cell growth.
Methods:
The IC50 values of select chemotherapeutic agents were measured using four human neuroblastoma cell lines, and they were calculated using GeneData Screener Condoseo software. Antiproliferative effects of pralatrexate were evaluated using the Cell Titer Glo or CCK8 assay. Adherent and non-adherent colony formation assays with pralatrexate were used to determine in vitro cell growth measures. Cell cycle arrest and cellular apoptosis were demonstrated by flow cytometry. Myc expression was examined by western blot, and SLC19A1 mRNA was examined by Q-pcr.
Results:
Treatment with pralatrexate in all four human neuroblastoma cell lines blocked cell growth in 2D and 3D culture conditions, and inhibited cell viability in a time-dependent manner. Pralatrexate-induced apoptosis was confirmed by caspase-3/7 activation and PARP cleavage. The IC50 of pralatrexate was 10-fold lower in all 4 neuroblastoma cell lines when compared to the IC50 values of methotrexate, another folate analog metabolic inhibitor. SLC19A1 mRNA expression did not change under pralatrexate treatment, whereas Myc expression was affected by pralatrexate in neuroblastoma cells.
Conclusion:
Pralatrexate demonstrated effective inhibition of neuroblastoma cell growth and viability. Further, the IC50 of pralatrexate was significantly lower than methotrexate, a drug with high levels of toxicity in neuroblastoma patients, suggesting pralatrexate may be a safer alternative to methotrexate. Our findings support the further clinical development of pralatrexate, alone or in combination with certain chemotherapy drugs and targeted agents, as a potential therapeutic strategy for the treatment of high-risk neuroblastoma.
E. J. Onufer1, J. Ou1, C. Courtney1, S. Sutton1, R. Czepielewski2, Y. Han2, G. Randolph2, B. W. Warner1 1Washington University in St. Louis,Surgery,St. Louis, MO, USA 2Washington University in St. Louis,Immunology,St. Louis, MO, USA
Introduction: Intestinal failure-associated liver disease (IFALD) is a major morbidity associated with short gut syndrome (SGS) and negatively impacts survival. The development of IFALD begins with steatosis and progresses to fibrosis, ultimately leading to cirrhosis. Given this pathology, we sought to determine if liver injury after small bowel resection (SBR) could be prevented in a unique mouse strain that is obesity-resistant (129S1/SvImJ).
Methods: Using our previously characterized TPN-independent model of resection-associated liver injury, C57BL/6 and 129S1/SvImJ mice underwent a 50% proximal SBR or sham operation. Body composition was assessed at baseline and 10-weeks post-operatively. At post-operative week 5 and 10, we also quantified serum triglyceride, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels. Liver triglyceride levels were determined after homogenization with lipid extraction and normalization to hepatic protein concentration.
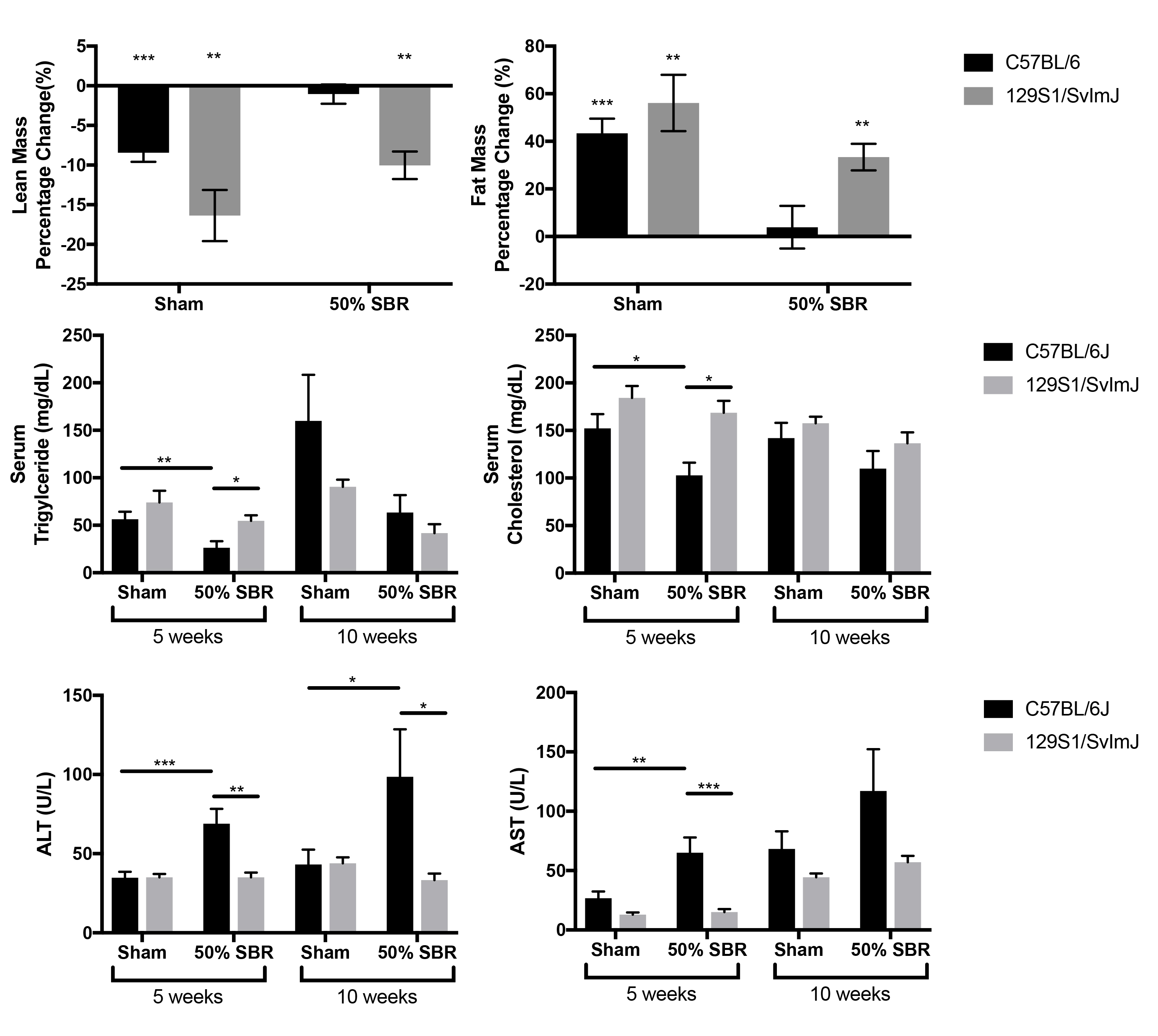
Results: Body composition from week 10 to baseline revealed a significantly greater decrease in lean mass with no difference in fat mass in 129S1/SvImJ compared to C57BL/6 mice in both operative groups. Serum triglyceride and cholesterol levels were significantly elevated in 129S1/SvImJ SBR mice at 5 weeks compared to C57BL/6, with no difference at 10 weeks. Hepatic triglyceride levels were no different between mouse strains. Serum ALT and AST were significantly elevated in C57BL/6 SBR mice compared to sham mice at 5 and 10 weeks; these effects were mitigated in 129S1/SvImJ mice. (Figure 1; *p<0.05, **p<0.01, ***p<0.0005).
Conclusion: Despite high levels of serum lipids,129S1/SvImJ mice do not develop liver inflammation and injury after SBR, unlike C57BL/6 mice. Further studies are needed to understand the mechanism behind resistance to IFALD development in 129S1/SvImJ.
A. Zhang1, P. Lu1, M. Wang1, E. M. O’Hare1, S. D. Brosten1, H. Ding3, C. L. Sears3, J. R. White4, D. J. Hackam1, S. M. Alaish1 1The Johns Hopkins University School Of Medicine,Surgery,Baltimore, MD, USA 3The Johns Hopkins University School Of Medicine,Medicine,Baltimore, MD, USA 4Resphera Biosciences,Baltimore, MD, USA
Introduction: Short bowel syndrome (SBS) is defined as massive small bowel loss resulting in malabsorption and an intestinal dysbiosis, a pathologic alteration in the microbiota. Lipocalin 2 (LCN2) is an antimicrobial protein, which reduces the availability of iron to proinflammatory bacteria and thus acts as a bacteriostatic agent. We hypothesized that the absence of LCN2 in SBS would result in greater relative amounts of pathologic bacteria and worse intestinal adaptation.
Methods: Under an ACUC-approved protocol, we performed 75% small bowel resection (SBR) or sham (SHA) operation on C57BL/6J wild type (WT) and LCN2 knockout (LCN2 -/-) mice. Body weight of the WT and LCN2 -/- mice were measured daily after SBR or sham operation. Intestinal tissue and cecal contents were collected on post-operative day 7 after euthanasia. Fecal DNA were isolated from cecal contents, 16s rRNA gene sequencing were performed and analyzed for all taxonomic groups on WT and LCN2 -/- mice. A 4% fecal slurry created with cecal contents from either WT or LCN2 -/- mice that previously underwent 75% SBR was gavage fed (fecal transplant) into germ-free mice. Body weight of the germ-free mice were measured before fecal transplantation and on post-transplant day 7 before euthanasia. Intestinal barrier integrity was assessed by permeability to FITC-Dextran in the serum following gavage feeding. The length of villi was assessed through histology. Statistical analysis was performed using ANOVA with p<0. 05 considered significant.
Results: A pronounced intestinal dysbiosis was observed in WT mice after 75% SBR, which was evidenced by significant relative increases of pro-inflammatory Proteobacteria and deceases of healthy Firmicutes and Bacteroidetes. Contrary to our hypothesis, LCN2-/- SBR mice had less pro-inflammatory Proteobacteria, and normal amounts of healthy Firmicutes and Bacteroidetes. Furthermore, LCN2-/- SBR mice had lower intestinal permeability and greater intestinal adaptation as compared to WT SBR mice. Germ-free mice that received fecal matter from LCN2 -/- SBR mice (GLR) gained 6% of their body weight and had decreased intestinal permeability and longer jejunal villi; whereas, germ-free mice that received fecal matter from WT SBR mice (GWR) lost 3% of their body weight and had increased intestinal permeability and shorter villi by comparison.
Conclusion:The presence of LCN2 results in a more pathologic intestinal dysbiosis in our mouse model of SBS. This altered microbiome leads to increased intestinal permeability and reduced adaptation. Inhibition of LCN2 may be a novel therapeutic target to improve intestinal adaptation and enteral tolerance in SBS patients.
C. H. Quinn1, R. Uhlich1, A. M. Beierle1, R. Marayati1, J. E. Stewart1, J. M. Coleman2, V. R. Atigadda3, G. K. Friedman4, E. A. Beierle1 1University of Alabama Birmingham,Division Of Pediatric Surgery, Department Of Surgery,Birmingham, ALABAMA, USA 2University Of Alabama at Birmingham,Department Of Neurosurgery,Birmingham, Alabama, USA 3University Of Alabama at Birmingham,Department Of Dermatology,Birmingham, Alabama, USA 4University Of Alabama at Birmingham,Division Of Pediatric Hematology Oncology, Department Of Pediatrics,Birmingham, Alabama, USA
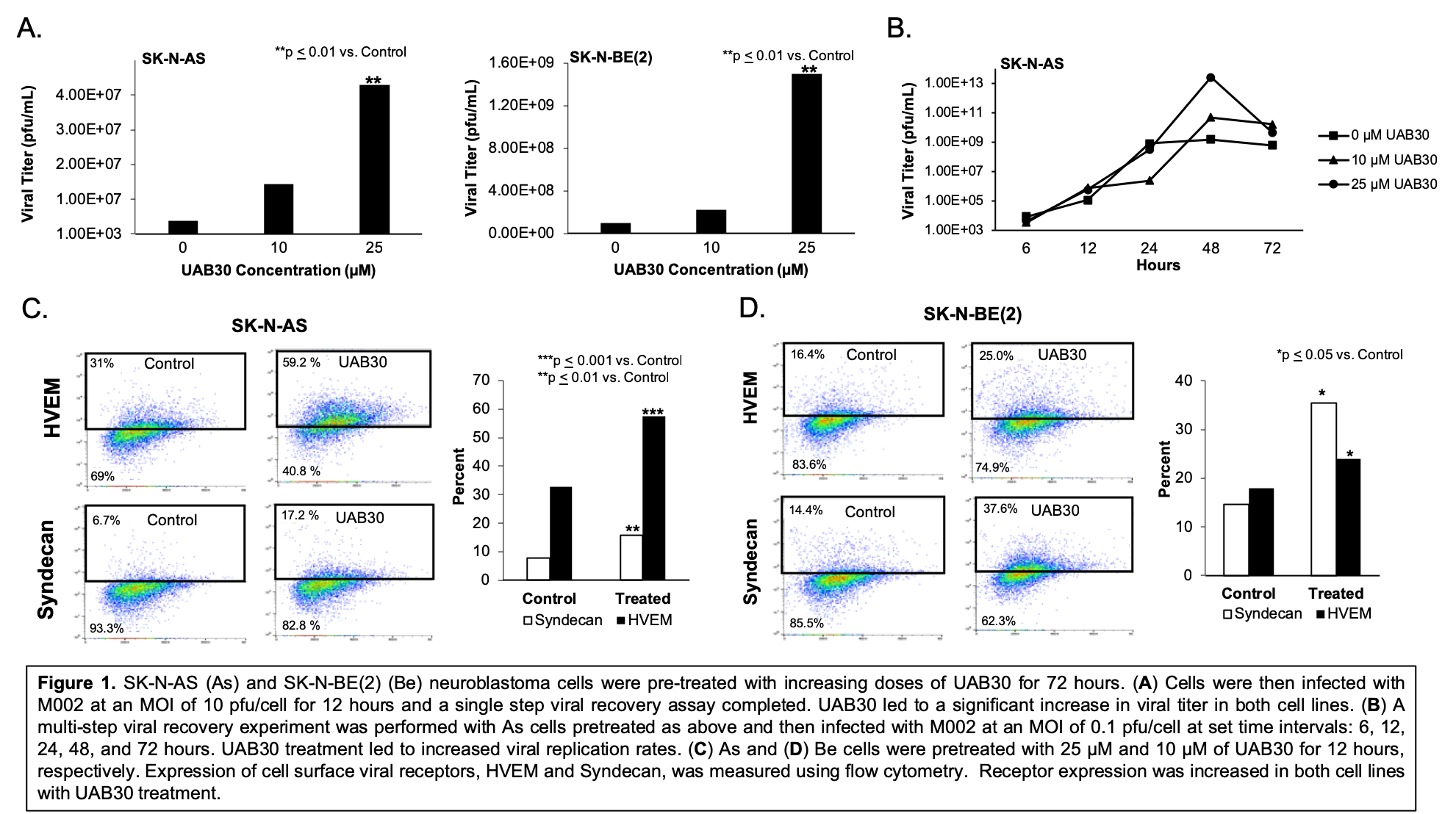
Introduction: Oncolytic virotherapy is a promising emerging therapeutic option for difficult to treat cancers. Combination approaches to maximize the oncolytic effect are being explored. Neuroblastoma, a solid tumor of the nervous system, is responsible for over 15% of pediatric cancer deaths. We have demonstrated significant neuroblastoma oncolysis with genetically engineered oncolytic Herpes Simplex Virus (oHSV) M002. Current therapy for neuroblastoma includes treatment with retinoic acid (RA) to stimulate tumor cell differentiation. We have found that UAB30, a novel RXR agonist, has similar cell differentiating properties as RA and is comparably efficacious in preclinical studies with neuroblastoma. We hypothesized that UAB30 would alter the tumor cell profile through alternate gene expression and increase tumor susceptibility to M002, thereby enhancing the efficacy of these potential neuroblastoma treatments.
Methods: M002 is a genetically engineered oHSV with deleted copies of both ?γ134.5 loci and UAB30 is a 9-cis-RA analogue. Neuroblastoma cell lines SK-N-AS (As) and SK-N-BE(2) (Be) were used. Single step and multistep viral recovery assays were performed following 72 hour pre-treatment with increasing doses of UAB30 to investigate M002 total viral production and replication rates, respectively. Flow cytometry was utilized to analyze expression of cell surface viral receptors following UAB30 pre-treatment. Cell viability studies were measured with alamarBlue® and synergy analyzed using the method of Chou and Talalay.
Results: Increasing doses of UAB30 significantly increased M002 viral titer (Figure 1A). 25 µM of UAB30 resulted in significantly increased M002 replication over time (Figure 1B). Cell surface HSV entry receptors, Syndecan and HVEM, were upregulated following UAB30 treatment (Figure 1C, D). In vitro studies with As cells treated with M002 and 25 µM of UAB30 resulted in a 7.4-fold decrease in the lethal dose required to kill 50% of cells compared to M002 alone (0.12 plaque forming units (PFU)/cell and 0.89 PFU/cell, respectively), indicating synergy.
Conclusions: UAB30 effectively increased M002 viral titer and replication. UAB30 combined with M002 resulted in significantly more neuroblastoma cell death compared to M002 alone. UAB30 increased the expression of several HSV cell entry receptors, providing a potential mechanism for the synergy seen between the agents. These studies suggest that UAB30 and M002 together may provide improved efficacy in neuroblastoma and validate the need for continued investigation.
S. I. Al-Juboori1, E. Dobrinskikh2, M. Oria3, J. L. Peiro3, A. I. Marwan1 1University Of Colorado Denver,Division Of Pediatric Surgery/Department Of Surgery,Aurora, CO, USA 2University Of Colorado Denver,Department Of Medicine,Aurora, CO, USA 3University Of Cincinnati,Division Of Pediatric Surgery,Cincinnati, OH, USA
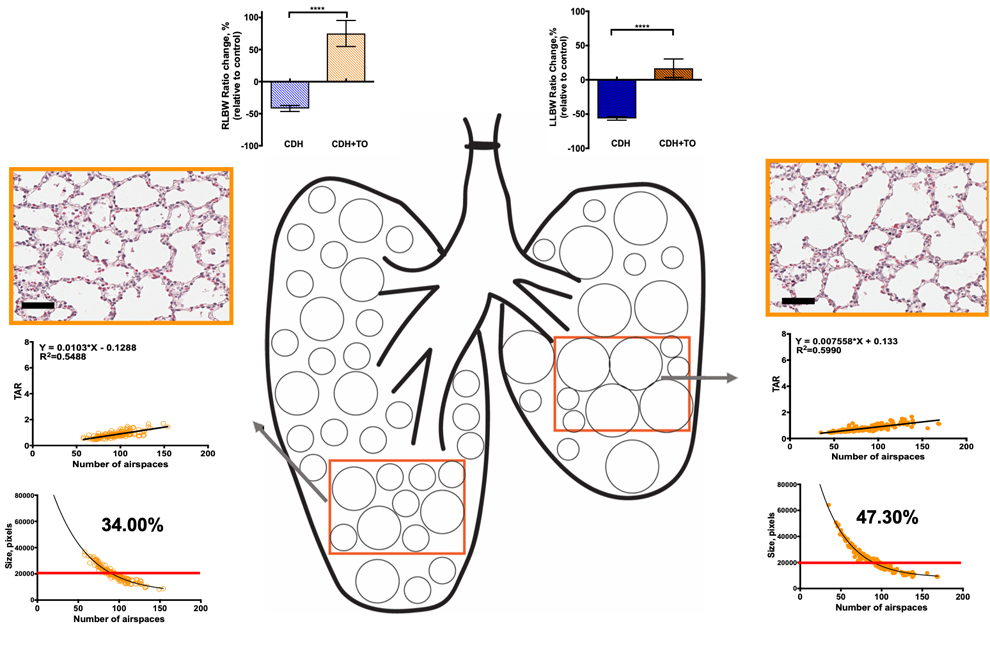
Introduction: Fetal Tracheal Occlusion (TO) is an experimental therapeutic approach to stimulate fetal lung development in the most severe Congenital Diaphragmatic Hernia (CDH) cases. Our laboratory has demonstrated a unique heterogeneous topological morphometric response of rabbit fetal lungs following 4 days of tracheal occlusion, namely: regions with control-like airspaces and others with enlarged airspaces. Currently, it is unknown whether TO in the setting of CDH will result in a similar heterogeneity. The aim of this study is to examine the heterogeneous fetal pulmonary parenchymal response following TO in the rabbit left-sided CDH model using morphometric analyses.
Methods: Fetal rabbits at 25 days gestation underwent surgical creation of left CDH followed by TO at 27 days and harvest on day 30. Lung-to-Body Weight Ratios (LBWRs) and their corresponding percent change relative to control counterparts, ΔLBWRs, were calculated for total lung weight, as well as left and right lung weights separately. In order to examine any heterogeneity in the pulmonary growth response in CDH model after TO, morphometric analyses, i.e., Tissue-to-Airspace Ratios (TARs), and the number and size of airspaces, were performed to evaluate parenchymal structural changes in control, CDH, and CDH+TO right and left lungs.
Results: Right and left lungs had a heterogeneous pulmonary growth response in CDH and after TO. The relative percent growth of the right lungs in CDH+TO was higher compared to the left lungs in the left-sided CDH model. Morphometric analyses revealed that right and left lungs were different at baseline and had heterogeneous TARs, in addition to size and number of airspaces within and between the lungs in the CDH and CDH+TO experimental groups. CDH right lungs had at least two distinct airspace populations, control-like, and larger TAR (smaller airspaces), unlike CDH left lungs where only one population was observed. TO resulted in the appearance of smaller TARs (enlarged airspaces) in both, right and left CDH+TO lungs. Furthermore, TARs were linearly and positively correlated with the number of airspaces in the pulmonary parenchyma of control, CDH, and CDH+TO animals. Whereas the number of airspaces and their respective average sizes exhibited an inverse nonlinear (exponential) relationship.
Conclusion: We demonstrated for the first time that morphometric response of CDH fetal lungs to tracheal occlusion is heterogeneous. Right and left lungs respond differently in percent growth and parenchymal structures. Parenchymal heterogeneity may result in variable functional response of the respiratory acini and therefore the unpredicted and variable clinical outcomes to this therapeutic intervention in hypoplastic lungs.
M. P. Shaughnessy1, C. J. Park1, L. H. McCarthy1, N. A. Barry2, A. L. Goodman2, R. A. Cowles1 1Yale University School Of Medicine,Pediatric Surgery,New Haven, CT, USA 2Yale University School Of Medicine,Microbial Pathogenesis And Microbial Sciences Institute,New Haven, CT, USA
Introduction: Previous work demonstrated enhanced enterocyte proliferation and mucosal growth in gnotobiotic mice colonized with a limited microbiome, supporting the notion that the intestinal flora participates in mucosal homeostasis. Prior studies have also shown that treatment with broad spectrum enteral antibiotics results in near germ-free (GF) conditions in mice with conventional flora (CF). We hypothesized that limiting the intestinal microbiome with enteral antibiotics would result in ordered small intestinal mucosal growth in CF mice but similar treatment would have no effect in GF mice lacking a microbiome.
Methods: C57BL/6J CF mice and C57BL/6J GF mice were allowed ad libitum access to either an antibiotic solution (Ampicillin, Ciprofloxacin, Metronidazole, Vancomycin, Meropenem) mixed in artificial sweetener (n= 3CF, 6GF) or artificial sweetener alone (n=3CF, 6GF). After two weeks, segments from the proximal, middle, and distal small intestine were harvested, fixed, sectioned and stained with H&E. Villus height (VH) was measured and mucosal surface area (MSA) was calculated. Cellular composition of villi and crypts was investigated by standard immunofluorescent staining for chromogranin A and lysozyme. Enterochromaffin cells (ECC), Goblet Cells (GC), and Enterocytes (EC) were counted and calculated as a percentage of total cells per villus, and number of Paneth Cells (PC) per crypt was assessed. Data were analyzed with Student’s t-test and significance assumed for p<0.05.
Results: Antibiotic-treated CF (Abx-CF) mice had taller villi and greater MSA in the proximal, middle, and distal small intestine when compared to vehicle-treated CF mice (p<0.0001 for all regions). Conversely, antibiotic-treated GF (Abx-GF) mice demonstrated no significant increase in VH in any segment of the small intestine when compared to vehicle-treated GF mice. When combined, there was no difference in total mean VH between Abx-GF and vehicle-treated GF controls (p=0.77). Abx-CF mice had an increase in total cells per villus along the entire small bowel (p<0.003). The proportion of EC in villi was unchanged in the middle and distal small intestine (p>0.05), with a small decrease in the proximal small intestine in Abx-CF. The proportion of ECC and GC in villi and PC in crypts was unchanged in Abx-CF mice compared to vehicle-treated CF mice (p>0.05).
Conclusion: Enteral administration of broad-spectrum antibiotics to mice with a conventional microbiome stimulates ordered small intestinal mucosal growth, resulting in taller villi with retention of normal cellular composition. Mucosal growth was not seen in germ-free mice treated with antibiotics, confirming that the microbiome is likely the target for the stimulatory effects of antibiotics on intestinal mucosal growth. The relationship between the microbiome and mucosal growth warrants further study and may provide guidance for development of future therapies for patients with malabsorptive disorders.
K. C. Ott1, M. Oria2, C. R. Redden1, H. K. Kang1, L. E. Turner1, A. A. Alhajjat1, S. Duru2, J. L. Peiro2, A. F. Shaaban1 2Center for Fetal and Placental Research,Division Of Pediatric General And Thoracic Surgery, Cincinnati Children’s Hospital Medical Center,Cincinnati, OH, USA 1Center for Fetal Cellular and Molecular Therapy, Ann and Robert H. Lurie Children’s Hospital,Department Of Surgery, Northwestern University Feinberg School Of Medicine,Chicago, IL, USA
Introduction: Neural tube defects (NTDs) are severe birth defects that originate during embryonic development when the neural tube fails to close completely. Stimulation of the maternal immune system in mice and humans reduces a wide variety of fetal irregularities suggesting that there is maternal immune surveillance of fetal anomalies such as NTDs. Given that the vast majority of maternal lymphocytes at the maternal-fetal interface are NK cells, we hypothesized that maternal NK cells are involved in protection against the development of NTDs in utero.
Methods: To challenge this hypothesis, we utilized a clinically-relevant murine model of valproic acid (VA) induced fetal neural tube teratogenesis with or without loss of maternal NK function. After pregnancy confirmation, groups of dams received either a depleting dose of an anti-NK cell mAb (PK136) or saline control on E7. VA-induced teratogenesis was performed on E8. All litters were then harvested on E14 and the litter size and prevalence of NTDs compared between the groups. The experiments were performed in both B6 (inbred) and CD1 (outbred) models.
Results: We found that 100% of the litters of NK cell-depleted dams (B6 and CD1) were affected by fetal NTDs. This was significantly higher when compared to litters of saline-treated dams (B6 = 60%; CD1 = 73%; p<0.05). Litter sizes were not significantly affected by the NK cell depletion (B6: 7.9 vs 8.6, p=NS; CD1: 12.9 vs 13.2, p=NS).
Conclusion: From these results, we conclude that maternal NK cells provide protection from the development of NTDs within the fetus. Further studies are needed to define the mechanisms by which maternal NK cells provide surveillance and protection from fetal teratogenesis.
M. Oria1, B. Pathak1, K. Bakri1, J. L. Peiro1 1Cincinnati Children’s Hospital Medical Center (CCHMC),Division Of Pediatric General And Thoracic Surgery,Cincinnati, OH, USA
Introduction: During spinal cord development, neural progenitor cells (NPC) generate three major cell lines; neurons, oligodendrocytes and astrocytes at precise time and position. Normally, neurogenesis occurs in early embryonic stage and astrocytes and oligodendrocytes later. However there have been studies indicating of astrocyte differentiation in early embryonic stages of spina bifida aperta. To date the pathophysiological mechanisms related to astrocyte differentiation with reactive astrogliosis of spina bifida aperta are poorly understood. In our study, we characterize the development of reactive astrocytes that differentiates from Pax6 and Olig2 neural progenitor cells.
Methods: Twenty fetal rat spinal cords from retinoic-acid (RA) induced spina bifida and other 6 from sham controls were analyzed at three gestational times (E15, E17, and E20).
Differentially expressed genes were determined using standard RNA sequencing approach and PT-qPCR for not only the transcription factors Pax6, Olig2 and Nkx2.2, but also the HLH transcription factors, Mash1, Ngn2, ID1 and ID2 that regulate the spatiotemporal neurogenesis and gliogenesis.
Histological analysis using specific antibodies for NPC (Pax6, NKX2.2 and Olig2) and astrocytes (GFAP) were used to quantify the NPC and to identify the NPC differentiating into astrocytes
Results: Patterning factor Pax6, NKX2.2 and Olig2 were downregulated in spina bifida animals at the 3 gestational times (E15, E17 and E20) in the RNAseq data and validated by RT-qPCR compared to non-spina bifida animals. GFAP gene expression was upregulated in spina bifida animals compared to normal animals during gestation. We have identified the presence of Olig2+ and Pax6+ multipotent progenitor cells in GFAP+ reactive astrocytes during gestation and increased numbers in the spinal cord tissue exposed to amniotic fluid in the spina bifida rats. Downregulation of patterning factors in the NPC and upregulation of astrogenic signaling pathway via BMP2 and 4 suggest early differentiation of the NPC into astrocytes in spina bifida aperta.
Conclusion: Together, our observations demonstrate altered premature astrogenesis affecting normal neurogenesis and oligodendrogenesis contributing to injury, and neural loss in spina bifida aperta after birth.
M. Sampah1, C. P. Sodhi1, M. L. Kovler1, A. J. Gonzalez Salazar1, M. R. Ladd1, H. Jia1, P. Lu1, Y. Yamaguchi1, W. Fulton1, T. Prindle1, S. Wang1, D. J. Hackam1 1The Johns Hopkins University School Of Medicine,Division Of General Pediatric Surgery,Baltimore, MD, USA
Introduction: Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants. Our lab and others have shown that NEC is mediated by excessive activation of Toll-like receptor 4 (TLR4), which is upregulated in the premature intestinal epithelium. While mice who are genetically deficient in TLR4 are protected from NEC, less is known about the downstream consequences of TLR4 activation that lead to intestinal injury in this disease. Among the many injurious cytokines produced in response to TLR4 activation in the premature intestine, TNF-alpha is one of the strongest pro-inflammatory effectors. Therefore, to better understand the downstream effects of TLR4 activation in the intestine, we sought to determine if mice lacking TLR4 were protected from intestinal injury caused by exposure to TNF-alpha.
Methods: NEC was induced in 7d old wild-type (TLR4WT) and TLR4 knockout (TLR4-/-) mice (equal number male and female) through four days of gavage-fed formula mixed with NEC bacteria and repeated exposure to brief periods of hypoxia. NEC severity was assessed histologically using a validated NEC severity score and through measurement of gene expression of TNF-alpha by qRT-PCR of intestinal segments. To further understand the ensuing effects of TLR4 activation, juvenile TLR4WT and TLR4-/- mice were treated intraperitoneally with either murine TNF-alpha (5ug) or saline as controls (5 mice per group). After six hours, mice were sacrificed and ileal tissue was collected to assess for expression of the pro-inflammatory cytokines and necrotoptic markers including TNF-alpha, Lipocalin-2 and IL-1beta by quantitative RT-PCR.
Results:TLR4WT mice subjected to experimental NEC showed significantly higher histologic severity scores and TNF-alpha expression when compared to breast fed controls. In contrast, mice genetically deficient in TLR4 were protected from experimental NEC and did not have increased TNF-alpha expression. In contrast, following direct intraperitoneal administration of TNF-alpha, TLR4−/− mice were not protected from intestinal inflammation, and actually had elevated gene expression of Lipocalin-2 when compared to TLR4WT mice.
Conclusion:These findings demonstrate that while excessive TLR4 signaling in the premature intestinal epithelium is a requisite component in the pathogenesis of NEC, TLR4 deficiency is not protective against the subsequent inflammatory response that proceeds. Therefore, while TLR4 inhibition remains an important therapeutic target for NEC, mediating the downstream inflammatory response to TLR4 signaling in the premature intestine – including the possibility of direct TNF-alpha inhibition – offers novel potential approaches to treating this devastating disease.
M. P. Kallis1,2,3, B. Blank2,4, M. Symons2,5, B. M. Steinberg2,5, S. Z. Soffer1,3 1Donald and Barbara Zucker School Of Medicine at Hofstra/Northwell,Surgery And Pediatrics,Manhasset, NEW YORK, USA 2The Feinstein Institutes for Medical Research at Northwell Health,The Elemezzi Graduate School Of Molecular Medicine,Manhasset, NEW YORK, USA 3Cohen Children’s Medical Center at Northwell Health,Surgery,New Hyde Park, NY, USA 4Donald and Barbara Zucker School of Medicine at Hofstra/Northwell,Hempstead, NEW YORK, USA 5Donald and Barbara Zucker School of Medicine at Hofstra/ Northwell,Molecular Medicine,Manhasset, NEW YORK, USA
Introduction: Primary tumor excision is the mainstay of treatment for osteosarcoma (OS). However, surgical stress may promote metastatic growth, an effect known as surgery-accelerated metastasis. We have previously shown that surgical stress alters the proportion of macrophage populations within the lung to favor a pro-tumor macrophage phenotype. The transcriptional changes contributing to this phenotype are unknown.
Methods: Mouse OS cells (K7M2) were implanted into the tibia of BALB/c mice. Animals were then randomized into tumor-bearing or tumor-amputation groups (n=3-4 mice/group). The amputation group underwent amputation of the primary tumor-bearing limb at 1 week post tumor implantation. Both groups were sacrificed 4 weeks after tumor inoculation. Macrophages, defined as CD45+/F4/80+ double positive cells, were isolated from whole lung homogenate using fluorescence activated cell sorting (FACS). Mature macrophage RNA was isolated and reverse transcribed using a cDNA conversion kit and a PCR array examining genes related to cancer and immunity crosstalk was performed. Macrophage gene expression of experimental groups was compared to that of age-matched healthy controls.
Results: Implantation of a primary tumor alone resulted in a 5.8 fold increase in chemokine receptor type 4 (CXCR4) (p< 0.05). After surgical resection expression of CXCR4 increased 10.8 fold (p< 0.01). Chemokine receptor type 3 expression (CXCR3) decreased by 63 fold following implantation of a primary tumor (p > 0.01), and after surgical excision expression remained decreased by 11 fold compared to controls (P< 0.05). Signal transducer and activator of transcription 3 (STAT3) increased in expression 4 fold after implantation of tumor alone, and further increased after surgery to 5.25 fold (p< 0.05).
Conclusion: Implantation of a primary tumor increases expression of genes known to assist in migration, invasion, and adhesion in metastasis (CXCR4), as well as genes associated with immunosuppression and decreased tumor surveillance (STAT3). Tumor presence also results in decreased expression of a gene known to activate macrophage tumoricidal activity (CXCR3). Following surgical resection, the expression of these genes remain altered toward a pro-tumor and immunosuppressive phenotype compared to non-operated tumor-bearing mice, demonstrating the persistent tumorigenic environment in the enhanced metastatic niche post-operatively.
K. Marulanda1, A. Mercel1, M. Gambarian1, D. C. Gillis1, K. Sun1, M. Karver2, N. D. Tsihlis1, S. E. McLean1, M. R. Kibbe1 1University Of North Carolina At Chapel Hill,Department Of Surgery,Chapel Hill, NC, USA 2Northwestern University,Simpson Querrey Institute,Chicago, IL, USA
Introduction: Pulmonary hypertension (PH) is a highly morbid disease without an effective treatment. Our aim is to develop a systemically administered nanoparticle therapy that specifically targets the pulmonary vasculature. Angiotensin converting enzyme (ACE) is highly associated with PH pathogenesis, demonstrating increased expression in the diseased pulmonary vascular endothelium. To target ACE, self-assembled peptide amphiphile (PA) nanofibers are an ideal delivery vehicle, as they are readily modifiable, biocompatible, and can be re-dosed. We hypothesize that ACE-targeted PA nanofibers will localize to the pulmonary vasculature in a mouse model of chronic hypoxia.
Methods: Two ACE-targeted amino acid sequences, GNGSGYVSR (GNG) and RYDF, were covalently attached to a PA backbone. PAs were synthesized using solid phase peptide synthesis, then purified and characterized by high pressure liquid chromatography paired with mass spectrometry (HPLC-MS). The GNG- and RYDF-PA nanofibers were co-assembled using different ratios of backbone PA and fluorescently tagged PA. Conventional transmission electron microscopy (TEM) was used to assess nanofiber formation. Female and male C57BL/6J mice (8-10 weeks old) were exposed to chronic hypoxia (10% FiO2) for 3 weeks. Control mice were kept at room air (21% FiO2). To assess in vivo nanofiber localization, targeted nanofiber (10mg/kg) was administered to control and hypoxic mice via tail vein injection. Lungs were harvested after 30 minutes, and nanofiber fluorescence was quantified.
Results: HPLC-MS confirmed >95% purity of PAs, and TEM confirmed nanofiber formation for both ACE-targeted nanofibers. The mouse PH model was validated by observing pulmonary arterial muscularization per high power field on histology and elevated right ventricular systolic pressure with hemodynamic assessment. ACE immunostaining levels were 5-fold higher in the hypoxic versus control mouse lungs (3106 ± 287 vs. 644 ± 98 AU, n=3, p<0.0001), validating this protein as a useful target. After inducing PH in the mice, targeted nanofibers were injected systemically. Interestingly, the RYDF-PA nanofiber demonstrated extremely high (78-fold) binding affinity in hypoxic versus control lungs (390 ± 35 vs. 5 ± 2 AU, n=2-4/treatment group, p<0.0001) while the GNG-PA nanofiber demonstrated no difference in binding between the groups (15 ± 5 vs. 21 ± 5 AU, n=3-4, p=0.39). The pattern of binding of the RYDF-targeted nanofiber to the pulmonary vasculature had a similar distribution pattern as ACE immunoreactivity on fluorescent microscopy.
Conclusion: An ACE-targeted PA nanofiber was successfully designed and synthesized, and specifically localized to the pulmonary vasculature following intravascular administration in a mouse model of chronic hypoxia. Our findings lay the groundwork for incorporation of a therapeutic into the targeted nanoparticle to effectively mitigate pulmonary hypertension.
W. J. Melvin1, F. M. Davis1, C. Audu1, A. Joshi1, A. Obi1, B. Moore1, K. A. Gallagher1 1University Of Michigan,Ann Arbor, MI, USA
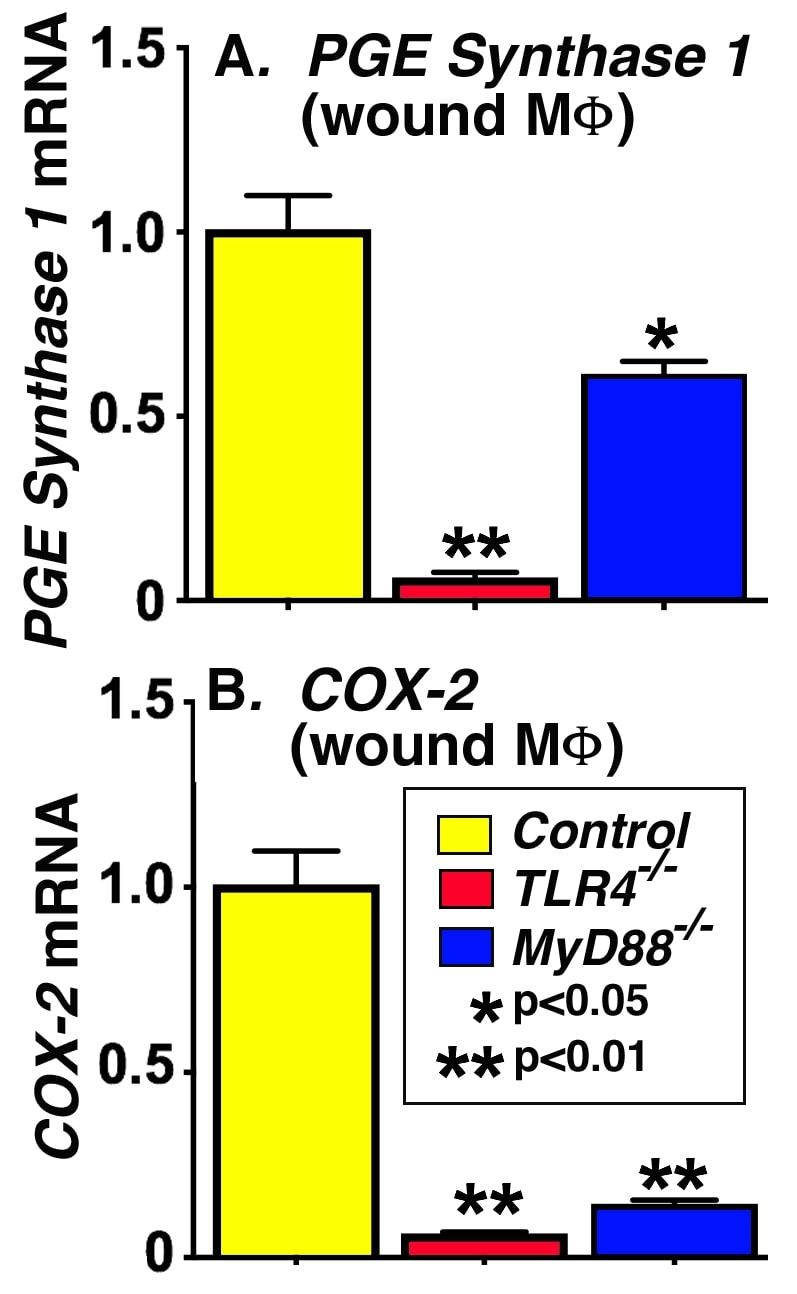
Introduction: Persistent uncoordinated inflammation is a hallmark of non-healing diabetic wounds but our understanding of this pathophysiology remains incomplete. Our recent work has identified that overproduction of prostaglandin E2 (PGE2) in wound macrophages (Mφs), chiefly driven by overexpression of the cyclooxygenase-2 enzyme (COX-2), is a key contributor to this pathologic inflammation that inhibits normal wound healing. The etiology of this overexpression is not yet elucidated. Since TLR4 signaling has been found to be a crucial pathway in Mφs for the inflammatory response, we hypothesize that TLR4 signaling in wound Mφs regulates COX-2 and PGE2 expression and hence, pathologic inflammation in diabetic wounds.
Methods: C57BL/6 wild type mice with TLR4-/- and MyD88-/- knockouts (n=15 / group) were wounded with a 6mm punch biopsy and wounds were harvested after 5 days. Wound Mφs (CD3-CD19-NK1.1-LY6G-CD11b+) were isolated and analyzed for PGE Synthase 1 and COX-2 expression by quantitative PCR.
Results: Wound Mφs from mice deficient in the TLR4 receptor and its immediate downstream signaling component, a myeloid differentiation factor, MyD88, demonstrate significantly (p<0.05) decreased COX-2 and PGE2 expression compared to control wound Mφs. Differences between groups were evaluated using ANOVA.
Conclusion: The TLR4 pathway appears to regulate COX-2/PGE2 expression in wound Mφs and may mediate inflammation in wounds. Targeting this pathway in a Mφ-specific manner has therapeutic implications for improved wound healing in diabetes.