A. Labora1, H. Lee2, K. Rashid2, T. Le2, C. Chan1, T. Yamao1, E. Abt2, J. Link1, A. Premji1, A. Creech2, L. Li1, N. Wu1, C. Radu2, T. Donahue1 1University Of California – Los Angeles, Surgery, Los Angeles, CA, USA 2University Of California – Los Angeles, Pharmacology, Los Angeles, CA, USA
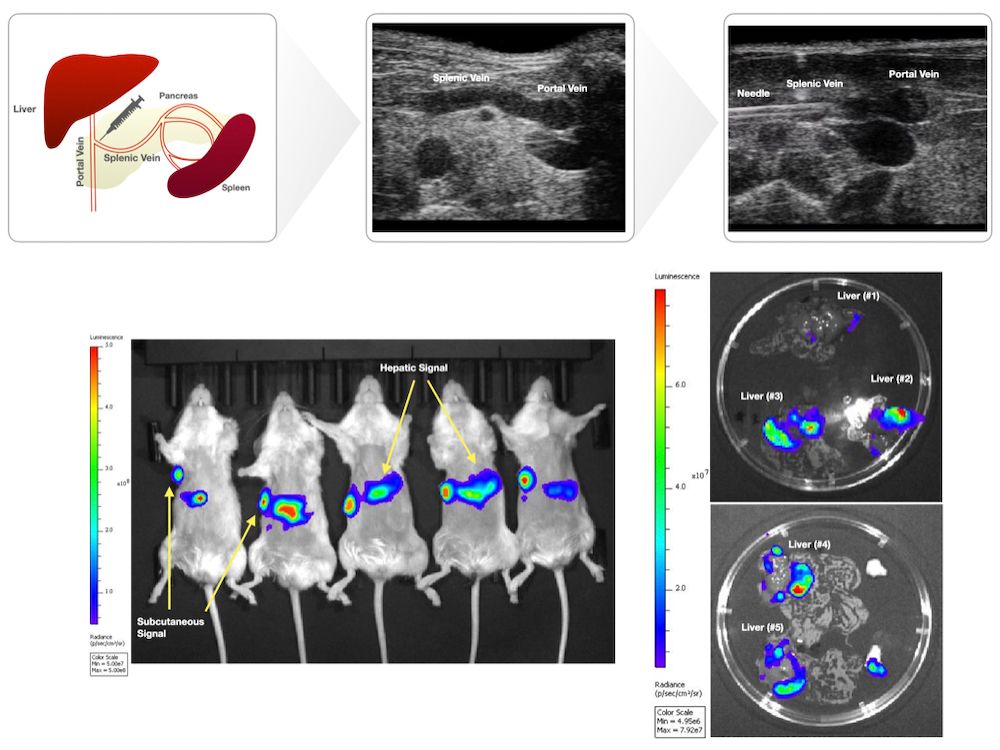
Introduction: The lack of biologically relevant preclinical models constitutes a major barrier to improving therapies for pancreatic cancer. Although most patients with pancreatic cancer present with metastatic disease, preclinical studies disproportionately rely on subcutaneous and orthotopic models. Previously described liver metastatic models such as the portal vein and intrasplenic injection methods employ open surgical procedures that are time-consuming and invasive. We report a novel technique to generate liver metastases via an ultrasound-guided splenic vein injection.
Methods: Ultrasound-guided splenic vein injection: Prior to injection, mice underwent hair removal. Mice were fasted for 12 hours prior to injection. Anesthetized mice were secured in the supine position on the pre-heated imaging platform. Ultrasound gel was applied, and the linear transducer (Vevo 2100) was used to locate anatomical landmarks (left kidney, stomach, spleen). A safe injection window whereby the splenic vein could be accessed at its confluence with the portal vein was established. Probe pressure was applied as needed to displace overlying viscera. The needle was positioned in-plane and advanced using toothed forceps to provide counter tension. The needle was advanced through the pancreas to access the splenic vein. Intravascular placement was confirmed by aspirating blood. Cells were subsequently injected into the vein. Following injection, the needle was slowly retracted under ultrasound guidance. Probe pressure was applied for several minutes, and the area was observed to ensure that no trauma had occurred. The mice were observed post-procedure to ensure adequate recovery. Animals: Female NSG mice, aged 8-to-10 weeks (N = 5). Cell Culture and Injection Preparation: HPAC cells expressing luciferase (HPAC-luc) were maintained in DMEM/F12 (1:1) with 10% FBS at 37°C in 5% CO2. HPAC-luc cells (1×106 cells) were trypsinized, washed thrice in PBS, and resuspended in PBS (20 μL for splenic vein injections, 100 μL for subcutaneous injections) prior to being loaded in 1 mL luer lock syringes with 30 G needles for splenic vein injections; 29.5 G insulin syringes were used for subcutaneous injections.
Bioluminescent imaging: Mice were injected intraperitoneally with 50 mg/kg D-Luciferin and imaged with the IVIS Lumina III imaging system. Data were analyzed using Living Image v4.5 software.
Results: Mice were imaged by BLI 22 days post-procedure and were found to have detectable BLI signal at both the subcutaneous site and hepatic region. Ex vivo BLI was performed on excised livers to confirm hepatic signal.
Conclusion: Injection into the splenic vein under ultrasound guidance is feasible and consistently generates liver metastasis.
S. R. Govindu1, A. P. Shah1, A. F. Espinoza1, R. Patel1, S. A. Vasudevan1, S. E. Woodfield1 1Divisions of Pediatric Surgery and Surgical Research, Michael E. DeBakey Department of Surgery, Pediatric Surgical Oncology Laboratory, Dan L. Duncan Cancer Center, Baylor College Of Medicine, Houston, TX, USA
Introduction:
Hepatoblastoma (HB) is the most common pediatric primary tumor of the liver. Relapsed and treatment refractory cases have a survival rate of less than 50% due to limited treatment options. Previous work showed NFE2L2/NRF2 hot-spot mutations in 11% of HB patients, all with high-risk tumors. NRF2 is a transcription factor that directly activates multiple target genes including NQO1, and carcinogenic effects of mutant NFE2L2 are dependent on mTOR. Previous literature suggests upregulation of phosphorylated mTOR in 96% of HB cases. Thus, we tested HB cell lines with the mTOR inhibitor sapanisertib to establish efficacy and mechanism of action.
Methods:
Expression of NQO1 was first assayed in HB cell lines (HepG2, HepT1, B6-2, HB17) with qRT-PCR experiments. Sapanisertib sensitivity of these cell lines were then tested with cytotoxicity (MTT) assays. Immunoblotting assays were used to assess changes in expression of total and phosphorylated mTOR and AKT with sapanisertib treatment.
Results:
HepT1 (NFE2L2 mutant), B6-2, and HB17 cell lines with high expression of NQO1 showed strong sensitivity with IC50 values of 0.08, 0.44, and 0.04 μM, respectively. HepG2 (NFE2L2 wild-type) with low expression of NQO1 showed resistance to sapanisertib up to 10 μM. Thus, sensitivity to sapanisertib correlated with NQO1 expression. Further studies with HepT1 cells treated with a combination of sapanisertib and cisplatin indicated that combinatorial therapeutic regimens can lead to a significant decrease in viability of NFE2L2 mutant cells. Immunoblotting showed decreased expression of phosphorylated AKT for HepT1 (NFE2L2 mutant) and no expression of phosphorylated AKT for HepG2 (NFE2L2 wild-type) treated with sapanisertib.
Conclusion:
Sapanisertib demonstrates strong in vitro efficacy in HB. This work suggests mTOR inhibition may act through a NRF2/NQO1 mediated mechanism. Further work with this drug may lead to clinical trials with HB patients.
A. Dedeilia1, K. Krause1, T. Sharova1, W. Michaud1, D. Liu2, G. Boland1 1Massachusetts General Hospital, Department Of Surgery, Boston, MA, USA 2Dana Farber Cancer Insititute, Department Of Medical Oncology, Boston, MA, USA
Introduction:
Although cutaneous melanoma has been the focus of multiple recent studies, mucosal (MM) and acral (AM) melanoma remain two rare (1-2% and 2-3% of melanomas respectively) and understudied forms of the same cell origin. They are more prevalent among women and non-Caucasian populations and have a higher rate of metastasis at presentation and worse prognosis and response to treatment. Thus, it becomes essential to investigate the biology, microenvironmental interactions and potential therapeutic candidates of MM and AM, to bridge the current evidence gap.
Methods:
A biobank of more than 30 MM and 30 AM were retrieved from a large cohort of melanoma patients. The tumor specimens are being studied via bulk sequencing, including targeted sequencing, whole exome sequencing (WES) and RNA sequencing (RNAseq) to identify somatic mutations and transcriptomic features of cell populations involved in tumorigenesis and responsiveness to therapy. Also, single-cell RNA sequencing (scRNAseq) performed on a subset of patients provides transcriptome information on individual cells and assists in the documentation of the expression of specific protein targets.
Results:
Pilot data demonstrates that MM cells express SOX10, a potential therapeutic target shared with cutaneous melanoma (CM). Furthermore, data shows that compared to CM, MM tumors lack memory/effector T cells and IFN-activated myeloid cells, which have been associated with successful checkpoint immunotherapy in melanoma, while they had high numbers of gamma/delta T cells, cytotoxic CD8 T cells, and macrophage/monocytes thought to create an immunosuppressive microenvironment. Ongoing efforts are focused on expansion and validation of the preliminary findings.
Conclusion:
This work provides a highly needed map of these rare cancers (AM and MM). Bulk sequencing sheds light to the intercellular microenvironmental interactions, and single cell RNA sequencing provides valuable information on the expression of candidate targets that will drive further research for the respective novel therapeutic targets for AM and MM.
G. Tushoski-Alemán1, K. Herremans1, S. Han1, S. Hughes1 1University Of Florida, Department Of Surgery, Gainesville, FL, USA
Introduction: Cytotoxic (CD8+) T-cells serve an essential role in the immune response to neoplasms. Assessment of intratumoral populations of CD8+ T-cells in pancreatic ductal adenocarcinoma (PDAC) have previously relied upon semi-quantitative assessment and remains largely unexplored in relation to overall survival. We hypothesized that CD8+ T-cell infiltration rates, measured quantitatively via a digital-pathology platform would associate with overall survival.
Methods: Fifty-five PDAC tumors and 28 non-malignant pancreatic tissues were assembled into a tissue microarray (TMA). TMA slides were immunohistochemically stained with anti-CD8 antibodies. Using the digital quantification software package (QuPath), we performed a quantitative measurement of the number of intratumoral CD8+ cells in PDAC. Overall survival analysis was performed using Kaplan-Meier method,
Results: CD8+ T-cells represented a mean of 2.22% of nucleated cells in PDAC tumors (Range 0.08% – 8.18%), while chronic pancreatitis samples had significantly fewer CD8+ cells (p=0.0001) with a mean of 0.80% (Range 0.07%-3.78%). Linear regression analysis of CD8+ cell percentages, splitting the cohort into 2 even groups at the median, revealed a correlation (R2=0.153, p=0.0032) between higher levels of CD8+ T-cells and overall survival. PDAC tumors with high levels of CD8+ cell infiltration were found to have significantly better survival than their CD8+ low level counterparts, with a median survival of 904 days compared to 237 days (p=0.0006).
Conclusion: We demonstrate that a quantitatively-determined percentage of tumor-infiltrating CD8+ T-cells in human PDAC significantly associates with superior survival in PDAC.
N. H. Goldhaber1, T. Dong2,4, A. T. Phung4, J. R. Shah4, C. Larson3, A. B. Sanchez2, O. Aisagbonhi5, B. Oronsky2, W. C. Trogler4, T. Reid3, A. C. Kummel4, S. L. Blair1 1University Of California – San Diego, Department Of Surgery, San Diego, CA, USA 2University Of California – San Diego, EpcientRx Inc, San Diego, CA, USA 3University Of California – San Diego, Medical Oncology, San Diego, CA, USA 4University Of California – San Diego, Chemistry And Biochemistry, San Diego, CA, USA 5University Of California – San Diego, Department Of Pathology, San Diego, CA, USA
Introduction:
Oncolytic viruses can selectively infect tumor cells potentially inducing selective tumor cell lysis and activation of an immune response that has the potential to turn a “cold tumor” (one not attacked by the immune system) into a “hot tumor” (one susceptible to attack by the immune system) by the release of immune-stimulatory cytokines and molecules. Turning an immunosuppressed “cold tumor” into a “hot tumor” by increasing immune cells inside an oncolytic virus infected tumor is a key to improving anticancer activity.
Adenoviruses (Ad) as an oncolytic viral therapy has shown therapeutic effects on local treatment of cancers in clinical trials. However, its more widespread use is hindered not only by the requirement of the tumor cells to have a coxsackievirus and adenovirus receptor (CAR) for effective infections but also by the requirement for local administration (injection into a tumor).
Methods:
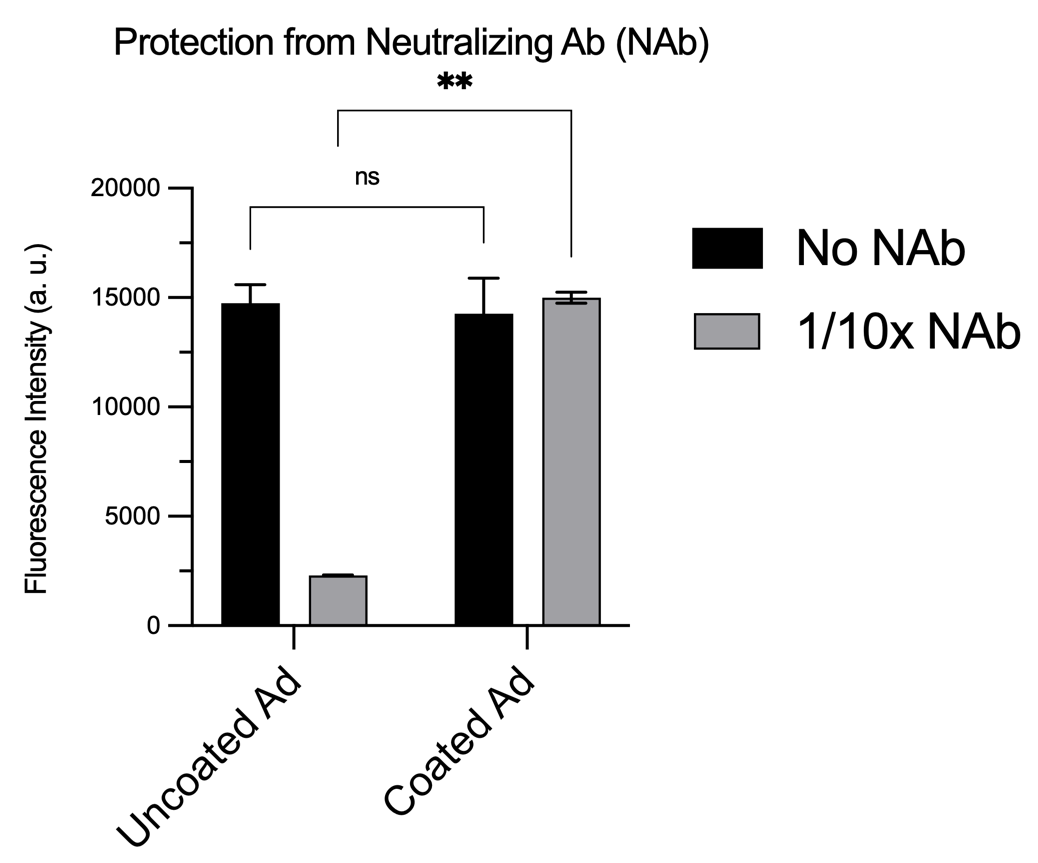
A liposome encapsulated adenovirus platform was developed to efficiently infect CAR-deficient cancer cells. The encapsulation of Ad by using cationic liposomes is the key to overcoming CAR-dependent infections and generating a strong immune response against infected tumor cells. The liposome encapsulation also provided a shield to allow Ad to escape from neutralizing antibodies (NAb) that may already exist in the blood. 90 nm diameter adenoviruses were encapsulated in 200nm extruded DOTAP-folate liposomes (Df) by simple incubation. The encapsulation process relies on simple charge interactions, and these encapsulated Ads (DfA) are able to infect CAR deficient tumor cells.
Results:
The encapsulated Ads (DfA) were tested in vitro first by incubating with neutralizing antibodies (NAb[BS1] ), and the results showed protection against the 1/10x neutralizing serum[BS2] (p value<0.005) which may further improve the efficacy of multiple doses of treatment.
The damage-associated molecular patterns (DAMPs), including extracellular ATP, HMGB-1 and CRT, confer an adjuvanticity to dying cancer cells, as they facilitate the recruitment and activation of immune cells, including APC, T cells and NK cells. The significant release of immunogenic molecules by DfA was assessed by transfecting CAR-deficient cells in vitro. There was significant more release of extracellular ATP (3200 vs 500 relative luminescence, p<0.05) and HMGB-1 (12 vs 3 ug/L, p<0.05).
Conclusion:
In conclusion, a DOTAP-folate encapsulated adenovirus has been shown to infect, lyse and induce a robust systemic immune response against CAR-deficient cancer cells. In the future, this construct can be utilized to improve efficacy of tumor vaccines strategies to prevent tumor recurrence.
H. Lindsay1, D. Foster1, M. Griffin1, J. Parker1, D. Delitto1, M. Longaker1, J. Norton1 1Stanford University, Department Of Surgery, Palo Alto, CA, USA
Introduction:
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with significant desmoplasia that limits immune and chemotherapy efficacy. Desmoplasia is caused by cancer associated fibroblasts (CAFs) that secrete collagen which cross link and result in a dense fibrous network. CAFs are the most populous cell type in PDAC and are an appealing target to improve anti-PDAC therapy. The purpose of this study is to identify targets to use for anti-CAF therapy to treat pancreatic ductal adenocarcinoma PDAC.
Methods:
To develop a murine model for PDAC the body of the pancreas was ligated and 25-50K KPC PDAC tumor cells were injected into the tail of the pancreas in C57 BL/6 mice. At 3 weeks post-injection, the tumor was harvested and fixed. The fixed tumor was embedded, sectioned, and stained using double antibody immunofluorescent (IF) staining.
Results:
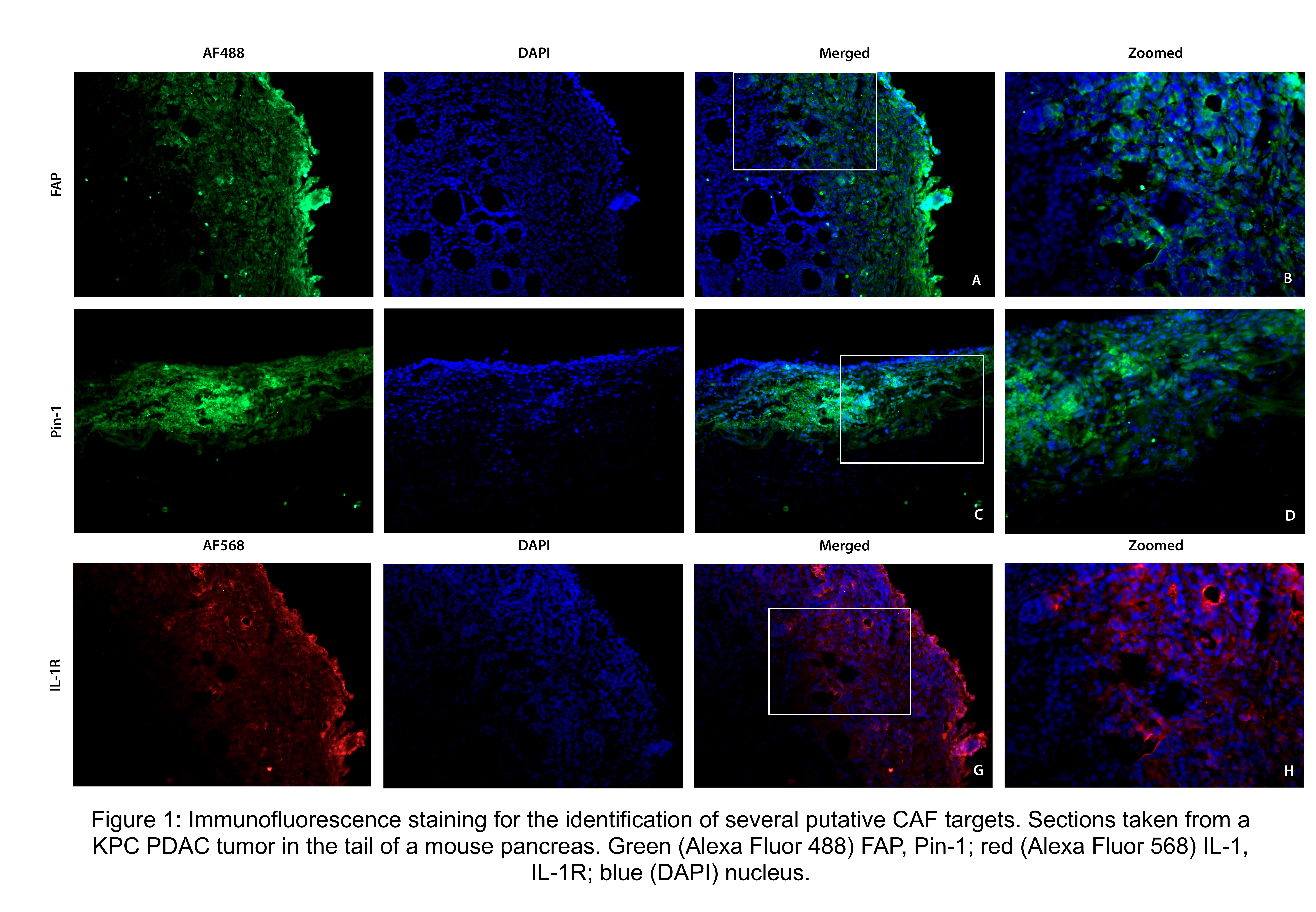
IF histology for Col-1 and α-SMA identified activated fibroblasts at the periphery of the tumor (not shown). IF demonstrated upregulated CAF markers of therapeutic interest; Fibroblast activation protein (FAP) (Figure 1, A and B), Peptidyl-prolyl cis/trans isomerase (Pin-1) (Figure 1, C and D), and interleukin-1 receptor (IL-1R) (Figure 1, G and H) had the most significant increased expression compared to non-specific binding control.
Conclusion:
The murine PDAC tumor shows prominent CAF expression suggesting excellent fibroblast recruitment and activation. IL-1R, Pin-1, and FAP, all potential targets for anti-CAF tumor therapies, are expressed near the surface of the tumor mimicking human PDAC. This model closely mimics human PDAC and will allow for analysis of in vivo anti-CAF PDAC treatments.
P. Kureti1, R. H. Patel1, A. F. Espinoza1, A. P. Shah1, A. Badachhape2, J. Epps3, S. R. Govindu1, P. Sumazin3, S. E. Woodfield1, S. A. Vasudevan1 1Baylor College Of Medicine, Pediatric Surgical Oncology Laboratory, Divisions Of Pediatric Surgery And Surgical Research, Michael E. DeBakey Department Of Surgery, Houston, TX, USA 2Texas Children’s Hospital, Singleton Department Of Radiology, Houston, TX, USA 3Baylor College Of Medicine, Department Of Pediatrics, Houston, TX, USA
Introduction: Hepatoblastoma (HB) is a rare childhood tumor of the liver that affects 2 in 1 million children a year. Most treatment options for this tumor are broad and limited. Patient-derived xenograft (PDX) models provide new avenues to treat hepatoblastoma by testing targeted agents with individual, well studied models. While models of multiple risk levels were tested, this paper focuses on two specific relapse models, HB28(+) and HB28(-), that were developed from the same patient but show contrasting characteristics.
Methods: Immunocompromised mouse livers were implanted with primary tumor samples and tumor growth was measured with MRI and ELISA. ELISA was used to evaluate amounts of human Alpha-FetoProtein (AFP) in the blood of these mice. Bulk and single cell RNA sequencing, mutation testing, Immunohistochemistry (IHC) for Hematoxylin and Eosin (H&E), Beta-Catenin, and Glypican-3 (GPC3), IHC slide analysis, and drug testing with Cisplatin were used to validate the tumors and show how the two models differed from each other.
Results: One relapse patient generated two differed PDX models that showed varying responses to the tests done above. HB28(-) seems to have no human AFP secretion in the blood and shows no expression of the gene. On the other hand, HB28(+) does show secretion of human AFP in the blood and expression of the gene. Both models retain all mutations found in the patient. IHC slide analysis indicated tumor growth in both models, with different histology patterns between the two models, and lung metastasis in both models. Drug testing of the models showed more resistance to Cisplatin with the HB28(+) model.
Conclusion: The two HB28 models showed unique and contrasting characteristics, and each model replicated sub-clones of the patient primary tumor, facilitating further drug testing. Future studies on the practicality of PDX models will guide treatment testing beforehand with real-time application to patients.
M. A. Enman1, S. Daniels1, U. Vaish1, T. Jain1, S. Iyer1, P. Sahay1, S. Giri1, A. Gutierrez-Garcia1, V. Dudeja1 1University Of Alabama at Birmingham, Surgery, Birmingham, Alabama, USA
Introduction:
The Aryl Hydrocarbon Receptor (AhR) is a ligand-activated transcription factor that is upregulated in pancreatic ductal adenocarcinoma (PDAC). Activation of AhR pathways promote the activation of pancreatic stellate cells (PSCs), pancreatic fibrosis, proliferation of cancer cells, as well as evasion of immune surveillance. We set out to study the effects of AhR inhibition on PSCs and cancer associated fibroblasts (CAFs).
Methods:
Pancreatic stellate cells (PSC) were isolated from the pancreas of AhR knockout (AhR -/-), AhR wildtype (AhR +/+), C57/BL6 mice, and plated on plastic. A pharmacologic inhibitor (Bay2416964) was used to inhibit AhR in BL6 PSCs and cultured for 24 hours. Conditioned media from KPC (LSL-KrasG12D/-;LSL-Trp53R172H/-;Pdx1-Cre) cells was added for 4 hours prior to trypsinization. From each of these PSCs, RNA was eluted, cDNA was created, and RT-PCR was performed to investigate PSC activation and CAF markers.
Results:
Genetic and pharmacological inhibition of AhR resulted in downregulation of downstream markers of AhR, including CYP1B1 and AhRr in the PSCs. Furthermore, markers of PSC activation such as aSMA, Col1a, LIF, IL-6, IL-11, and FAP were also downregulated in PSCs with AhR inhibition. FAP, a global marker for CAFs, was downregulated in PSCs with AhR inhibition co-cultured with conditioned KPC media.
Conclusion:
The pancreatic cell stroma is important in fibrosis and inflammation, as well as the development, growth, and proliferation in tumor cells. AhR-inhibited PSCs do not have the characteristic desmoplastic stroma that is seen in wildtype and BL6 PSCs. Our findings suggest that AhR inhibition has a negative effect on the activation and proliferation of PSCs, as well as a negative effect on the production of CAFs, which are crucial to the growth and proliferation of PDAC tumor cells.
J. T. George1,2 1Texas A&M University, Department Of Biomedical Engineering, Houston, TX, USA 2School of Engineering Medicine, Houston, TX, USA
Introduction:
Adjuvant immunotherapy has led to durable remission outcomes in many solid cancers. This therapy leverages billions of unique T-cells to confer immunity against foreign epitopes and tumor-associated antigens. Despite improvements in patient outcomes using T-cell immunotherapies, complete remission is limited by cancer immune evasion. Prior efforts have quantified the effect of immune system function of cancer control and immunotherapeutic efficacy[1,2]. We introduce a framework for quantifying the interaction between an adaptive immune system recognizing tumor-associated antigens (TAAs) on the surface of dominant cancer clones. We investigate the resulting evolutionary dynamics assuming tumors either passively evade recognition or actively escape adaptive immune detection. Using this framework, we represent cancer evasion on a spectrum of passive to active evasion. Our model serves as a first attempt at characterizing the extent of optimality of cancer evolution in the presence of the adaptive immune system.
Methods: We apply large-scale in-silico numerical simulations and discrete-time stochastic process theory to track the time dynamics of the number of detectable TAAs. Relevant modeling features include immune recognition and cancer evasion rates, and TAA availability, antigenicity, and accumulation rate. This model quantifies the likelihood and escape time of cancer immune evasion. Analytical equations are compared to largescale numerical simulations via a modified Gillespie algorithm to quantify model accuracy.
Results:
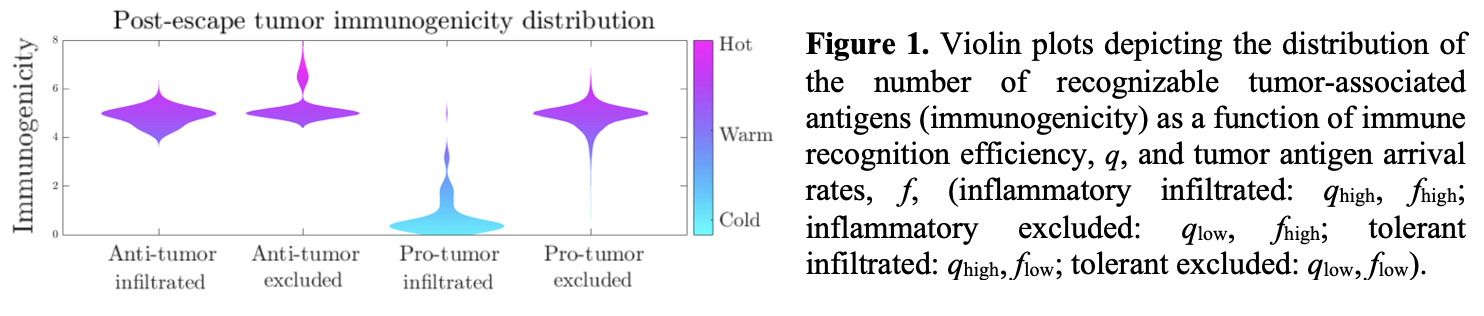
We correlates aggressiveness significantly with disease incidence across a variety of temporally varying recognition policies. Moreover, we find that the distribution of aggressive cancer immunogenicity following immune escape depends on the recognition efficiency of the adaptive immune system and the average arrival rate of new TAAs (Fig. 1). Substantial variety in evolutionary trajectories agree with prior clinical observations and together explain effects of the tumor-immune microenvironment on the generation of immunogenically hot or cold tumors.
Conclusion:
The development of a computational framework to track T-cell recognition (and cancer generation) of TAAs enables analysis of the likelihood and timing of immune escape, in addition to the predicted distribution of post-escape antigens. When applied to a variety of cancer microenvironments, our model can predict the clinically observed diversity in post-escape immunogenicity, which impacts the predicted efficacy of immune detection.
Acknowledgments:
JTG is a CPRIT Scholar in Cancer Research and is supported by CPRIT grant (RR210080).
[1] George JT, Levine H. Can Res 2020;80:811-819.
[2] George JT, Levine H. J Theor Biol 2018;458:148-155.
B. W. Armbruster1, A. F. Espinoza1, R. H. Patel1, S. A. Vasudevan1, S. E. Woodfield1 1Baylor College Of Medicine, Pediatric Surgical Oncology Laboratory, Divisions Of Pediatric Surgery And Surgical Research, Michael E. DeBakey Department Of Surgery, Houston, TX, USA
Introduction: Use of indocyanine green (ICG), a fluorescent dye, has recently gained popularity in surgical oncology. It can be used in surgical treatments of various forms of liver cancer through its ability to be specifically retained in malignant liver cells. Given this novel approach to distinguishing tumor tissue in the operating room, we sought to investigate whether ICG can be used to unambiguously identify malignant liver cells in in vitro studies.
Methods: First, we incubated cells in culture with ICG for 1 hour, washed the cells, and then imaged them with a fluorescence microscope with an ICG filter cube at two time points, immediately after washing and 24 hours after washing. We then mixed ICG avid (malignant liver cells) and non-ICG avid (fibroblasts) cells and repeated the ICG incubation, washing, and imaging. Second, we used flow cytometry with an APC-Cy7 laser to show ICG signal in ICG avid and non-ICG avid (neuroblastoma) cells incubated with ICG for 1 hour, washed, and then analyzed.
Results: We used fluorescence microscopy with a specific ICG filter to show that ICG fluorescent signal is highly present in malignant liver cells while notably absent from non-malignant liver cells and non-liver cells. This retention pattern was further corroborated with a mixed population of cells where malignant liver cells selectively took up and retained ICG, but no appreciable amount of ICG was present in the fibroblast population. Similar results were found with flow cytometry experiments with malignant liver cells and neuroblastoma cells with the liver cells clearly accumulating ICG while the neuroblastoma cells were negative. Further, we mixed the malignant liver and neuroblastoma cells and showed that the positive ICG signal is proportional to the number of malignant liver cells in a mixed population.
Conclusion: Taken together, this data shows that malignant liver cells in vitro specifically uptake and accumulate ICG over extended periods of time, in clear contrast to non-malignant liver cells and non-liver cells. Thus, ICG can be used in the laboratory setting to unambiguously identify liver tumor cells. In addition, this work paves the way to future studies that focus on understanding why ICG accumulates specifically in liver cancer cells.
A. K. Coley1, M. J. Raabe2, C. Lu2, A. Pankaj2, B. Patel2, I. Bhan3, C. R. Ferrone1, M. Aryee4, D. T. Ting2, J. W. Franses2 1Massachusetts General Hospital, Department Of Surgery, Boston, MA, USA 2Massachusetts General Hospital, Cancer Center, Boston, MA, USA 3Massachusetts General Hospital, Division Of Gastroenterology, Boston, MA, USA 4Dana Farber Cancer Insititute, Department Of Data Science, Boston, MA, USA
Introduction:
Hepatocellular carcinoma (HCC) tumorigenesis, progression, and recurrence is influenced by complex interactions between cancer cells, immune cells, and endothelial cells in the tumor microenvironment. Spatial transcriptomic techniques allow for in situ analysis of the complex cell-cell interactions in tumors within their usual spatial context. LIN28B is an RNA binding protein that plays critical roles in embryogenesis and oncogenesis via binding of the let-7 family of tumor suppressor microRNAs and other mRNA targets. LIN28B overexpression has been implicated in several cancer types as a driver of tumor development and metastasis. In HCC, high LIN28B expression is associated with the formation of poorly-differentiated tumors and worse overall clinical prognosis. In this study, we aimed to evaluate the impact of LIN28B expression on tumor-vessel interactions in HCC.
Methods:
We utilized the NanoString GeoMX Digital Spatial Profiler to profile the expression of ~1800 genes in formalin-fixed, paraffin embedded histological sections from 41 patients with HCC who underwent definitive treatment with surgical resection or liver transplantation. A total of 203 paired microscopic tumor (arginase positive) and vessel (CD31 positive) areas of interest (AOIs) were generated. Gene-specific oligonucleotide tags from each AOI were captured, sequenced on the Illumina NextSeq 500, and expression profiles were analyzed using custom computational pipelines. On sequential FFPE sections from the same patients, LIN28B IHC was performed and its expression was quantified using Halo digital image analysis software. Tumor AOIs and vessel AOIs derived from patients with high versus low LIN28B IHC expression were compared using differential expression analysis.
Results:
Differential expression analysis of tumor AOI and vessel AOI genes from LIN28B-high samples versus LIN28B-low samples led to the identification of multiple genes and pathways in both tissue compartments correlating with LIN28B cancer cell expression. Gene set enrichment analysis (GSEA) identified multiple enriched pathways.
Conclusion:
Spatial transcriptomic profiling enabled precise separation of linked, microscopic tumor and vessel subregions in LIN28B-high and LIN28B-low tumors that were defined by distinct gene sets. By preserving spatial relationships, spatial transcriptomic profiling can provide a rich understanding of cell-cell interactions in the tumor microenvironment that can generate novel, testable hypotheses for novel therapeutic targets and biomarkers in the HCC tumor microenvironment.
M. Maurer2, N. J. Skill1,2, Y. Bren-Mattison2, M. A. Maluccio2 1Louisiana State University Health Sciences Center, Interdisciplinary Oncology, New Orleans, LA, USA 2Louisiana State University Health Sciences Center, Surgery, New Orleans, LA, USA
Introduction: Neuroendocrine Tumors (NET) are a group of rare cancers originating from neuroendocrine cells within multiple organs. The evidence that supports medical decision making must consider the heterogeneity of these tumors, differences in biologic behavior between primary sites, and the moderate to high risk of developing metastatic disease over a patient’s lifetime. Based on preliminary omic studies and current clinical trials in other cancers, the enzyme 5′-nucleotidase (CD73) has been identified as a potential biomarker for patient stratification as well as likely treatment target for NET patients. CD73 is a cell membrane bound enzyme and has been linked to cancer development through the biosynthesis of adenosine from AMP. Increased expression of CD73 in tumors is associated with worse outcomes in stomach, pancreas, and lung cancers. Recently, small-molecule drugs that target CD73 directly or downstream of adenosine signaling pathway have been developed and are currently in clinical trials for other cancers.
Methods: Our laboratory operates one of the largest independent biorepositories for NET tissues and liquid biopsies. NET tumors and liquid biopsies from patients with NET were quantified for CD73, adenosine, and AMP. Tumor CD73 levels were quantified by Western blotting and ELISA (n=8). Plasma adenosine and AMP levels were quantified by HPLC/MS in plasma samples prior to surgery (N=12). Circulating CD73 levels were quantified by flow cytometry in peripheral blood mononuclear cells (PBMC) collected from patients with progressive NET post resection (N=25). Control PBMC and plasma samples were collected from living renal donors (LRD).
Results: AMP (the substrate for CD73) levels were markedly increased (>LRD+2SD) in plasma of NET patients compared to controls suggesting that this mechanism is a component of the metabolic alterations in NET. CD73 expression was increased in 20% of NET tumors congruent with elevated levels of adenosine (>LRD + 2SD) observed in plasma in 24% of patients from a separate cohort. Circulating CD73 levels are measurable and were increased (>LRD+2SD) within a subset of NET patients (8%) that are most relevant to our current prospective studies.
Conclusion: In a subset of NET patients, identified via increased tumor expression of CD73, increased circulating levels of CD73, and/or increased circulating levels of adenosine, targeting CD73 activity with emerging CD73 inhibitors may be reasonable to consider in certain patients. In this initial evaluation, up to 24% of patients would be potential candidates for CD73 targeted treatment. However, a caveat of this study is that the tumor and liquid biopsy analysis was not performed in matched samples from the same patient nor did we have enough events to link CD73 to cancer specific outcomes. Our prospective studies use the CD73 tumor expression to identify patients most relevant to further analysis and to better link the results to outcomes.
B. R. Herring1, C. MacVicar1, R. Guenter1, D. Dhall2, H. Chen1, G. Lee2, J. B. Rose1 1University Of Alabama at Birmingham, Surgery, Birmingham, Alabama, USA 2University Of Alabama at Birmingham, Pathology, Birmingham, Alabama, USA
Introduction: Immunogenic cell death (ICD) is often leveraged in immunotherapeutics. Calreticulin (CALR) is involved in the initiation of ICD, whereby upon sufficient insult to the cell it translocates from the endoplasmic reticulum to the plasma membrane and promotes phagocytosis of the damaged cell. Increased CALR portends improved outcomes for several cancers, but poor outcomes for others. However, no studies have examined the prognostic value of CALR for pancreatic neuroendocrine tumors (pNETs), which are often immunologically “cold.” Herein, we evaluate CALR as a prognostic biomarker for pNETs through immunohistochemistry (IHC) of tissue microarrays and retrospective chart review.
Methods: Following IHC of CALR, H-scoring was performed by a pathologist. Clinical variables analyzed included tumor grade, metastatic status (lymphatic and distant), and recurrence-free survival (RFS). 51 resected pNET samples were analyzed.
Results: CALR expression differed significantly between pNETs and normal pancreatic islets (p < 0.001), but not by tumor grade (p= 0.79), or either distant or lymph node metastatic status (p= 0.97; p= 0.09). CALR expression was not associated with tumor grade (p= 0.69), distant metastasis (p= 0.70), or lymph node metastasis (p= 0.40). However, CALR expression was positively associated with pNETs (compared to normal islets; p< 0.001). Furthermore, while there was a positive association between CALR expression and RFS, this relationship was not significant (p= 0.33).
Conclusion: While CALR expression is increased in pNETs, its influence on clinical outcomes remains unclear. Future studies will aim to examine these relationships in a larger cohort with more diverse clinical outcomes.
C. U. Ihemelandu1, A. Naeem2, O. Rodriguez2, C. Albanese2 1Georgetown University Medical Center, Surgical Oncology, Program In Peritoneal Surface Oncology, Washington, DC, USA 2Georgetown University Medical Center, Department Of Oncology And Pathology, Lombardi Comprehensive Cancer Center, Washington, DC, USA
Introduction: Conventional imaging modalities, such as CT, PET and MRI lack the sensitivity to detect and appraise tumor burden in patients with peritoneal metastasis of a colorectal cancer origin (CRC-PM). We propose utilizing circulating tumor exosome-associated miRNA as diagnostic/screening markers for early detection of CRC-PM recurrence and to guide targeted molecular therapy. Our hypothesis is that identification of specific miRNA associated with circulating CRC-PM tumor-derived exosomes represent early markers for detection of CRC-PM recurrence.
Methods: Exosomes were isolated from the serum of 6 CRC-PM patients, 4 healthy donors, and two CRC cell lines (SW620, HT116). Isolation of exosomes from serum samples was performed using the ExoQuick Plasma Prep kit. The size of the exosomes was verified using transmission electron microscopy. Exosomes were also characterized using markers CD63, HSP70, and CD9. RNA was isolated from exosomes using the miRNeasy Micro Kit. Qiagen miRNA library prep kit was used to prepare the miRNA library. RNAseq was performed on a HiSeq 2500 (Illumina, single-end 50-bp read length), using equimolar amounts for each sample.
Results: RNA sequencing analysis detected a total of 128 differentially expressed (DE) miRNA (p<0.01) in patients vs. healthy controls, and 203 miRNAs (p<0.01) in cell lines vs. healthy controls. Among the 128 DE-miRNA, expression levels of 56 DE-miRNA were downregulated, while those of 72 were upregulated in patients as compared to expression levels in controls (p< 0.05). Only one miRNA was differentially upregulated with FDR q<0.001. PCA analysis and unsupervised hierarchical clustering of the samples indicate a distinction among all groups i.e., patients, healthy, and cell lines. miR-3168 expression was noted to be significantly induced in cancer than in healthy controls with a log2 fold change of 3.8 (p<0.01, q<0.01) and in CRC cell lines as compared to healthy controls (log2 FC 1.2, p-value 0.02). Using MiRge 2.0, miRNA-3168 was annotated to biological functions such as response to stress, catabolic process, regulation of molecular function, and immune system processes. Furthermore, miRNA target filter analysis (Ingenuity Pathway Analysis @ Qiagen) revealed that miRNA-3168 is directly or indirectly involved in the regulation of well-studied CRC biomarkers EGFR and c-Met . Through its interaction with various molecules RPL17 (Ribosomal Protein L17), YBX1 (Y-Box Binding Protein 1), BDKRB2 (Bradykinin Receptor B2), miRNA-3168 is predicted to regulate the expression of EGFR and c-Met.
Conclusion: miRNA-3168 is significantly induced in CRC-PM patients and could serve as an additional biomarker in exosomes using liquid biopsy to detect and appraise peritoneal tumor burden.
A. E. Ramos1, S. Khan1, E. A. Novak1, H. L. Mentrup1, K. P. Mollen1 1University Of Pittsburgh, General Surgery, Pittsburgh, PA, USA
Introduction: Mitochondrial dysfunction is a hallmark of intestinal disease in IBD. Mitochondrial DNA (mtDNA) released into the cytosol can bind and activate the DNA sensor, Cyclic GMP-AMP Synthase (cGAS). Our lab has demonstrated cGAS activation to be protective during intestinal inflammation. However, the relationship of cGAS and mitochondrial health within the intestinal epithelium is unknown. We hypothesize that cGAS binds to mtDNA in intestinal epithelial cells and maintains homeostasis by regulating mitochondrial biogenesis and respiration.
Methods: We subjected cGAS KO, villincre;cGASfl/fl, lysmcre;cGASfl/fl, and WT mice to a 7-day model of DSS colitis. We performed electron microscopy (EM), WB analysis, qPCR, high resolution respirometry, and RNA sequencing analysis of the intestinal epithelium. We calculated disease activity index scores and performed colonoscopies on villincre;cGASfl/fl mice, lysmcre;cGASfl/fl mice, and their floxed controls.
Results: Our results demonstrate cGAS KO mice to have worsened mitochondrial health at baseline compared to WT mice as evidenced by EM. This discrepancy is exacerbated when cGAS KO and WT mice are subjected to the DSS induced colitis model with cGAS KO mice demonstrating near complete mitochondrial destruction. RNA sequencing analysis of the intestinal epithelium of cGAS KO mice subjected to DSS colitis showed a significant downregulation of mitochondrial respiratory pathways. To further evaluate mitochondrial respiration, we performed high resolution respirometry and found a decrease in complex I of the electron transport chain in cGAS KO mice subjected to DSS colitis. To evaluate mitochondrial biogenesis, we performed WB analysis which showed a decrease in two proteins involved in mitochondrial biogenesis, PGC1a and TFAM, in cGAS KO mice subjected to DSS. To assess for the role of cGAS in the intestinal epithelium, we subjected intestinal specific cGAS KO mice (villincre;cGASfl/fl) and myeloid specific cGAS KO mice (lysmcre;cGASfl/fl) to a 7-day DSS colitis model. Our data show that villincre;cGASfl/fl have significantly worsened intestinal inflammation compared to their floxed controls when subjected to DSS colitis whereas a myeloid-specific deletion of cGAS (lysmcre;cGASfl/fl) had no difference. Lastly, qPCR analysis for proinflammatory cytokines demonstrated higher levels of TNF-α and IL-6 in DSS-subjected villincre;cGASfl/fl mice compared to their floxed controls. This difference was not seen in the DSS-subjected lysmcre;cGASfl/fl mice compared to their floxed controls, suggesting the protective role of cGAS to be epithelial-specific.
Conclusion: We demonstrate intestinal epithelial cGAS deficiency to lead to worsened intestinal inflammation. Furthermore, our data show cGAS deficiency to be associated with decreased mitochondrial respiration and biogenesis.
M. Mirza1, J. Baechle1, P. Marincola1, J. Vaz2, A. Naveed1, D. Hanna1, M. K. Jolly2, K. Idrees1 1Vanderbilt University Medical Center, Division Of Surgical Oncology, Department Of Surgery, Vanderbilt-Ingram Cancer Center, Nashville, TN, USA 2Indian Institute of Science, Centre For BioSystems Science And Engineering, Banglore, BANGLORE, India
Introduction: Colorectal peritoneal metastases (CPM) portend worse clinical outcomes in comparison to hematogenous metastasis (HM). Yet the CPM disease biology remains poorly characterized. Herein, we aim to elucidate the role of collagen remodeling in CPM as a molecular pathway driving cancer progression and its interaction with TAMs in the tumor microenvironment (TME).
Methods: 70 matched cancer samples, surgically resected from 17 patients with colorectal metastasis were analyzed with bulk RNA-sequencing. Nanostring GeoMx digital spatial profiler (DSP) platform was used to for spatial localization and transcriptomics of 8 matched samples from 2 patients with colorectal metastasis. Gene Set Enrichment Analysis (GSEA) was used to calculate relative expression of collagen gene signature and the Ingenuity Pathway Analysis (IPA) was used for differential pathway analysis.
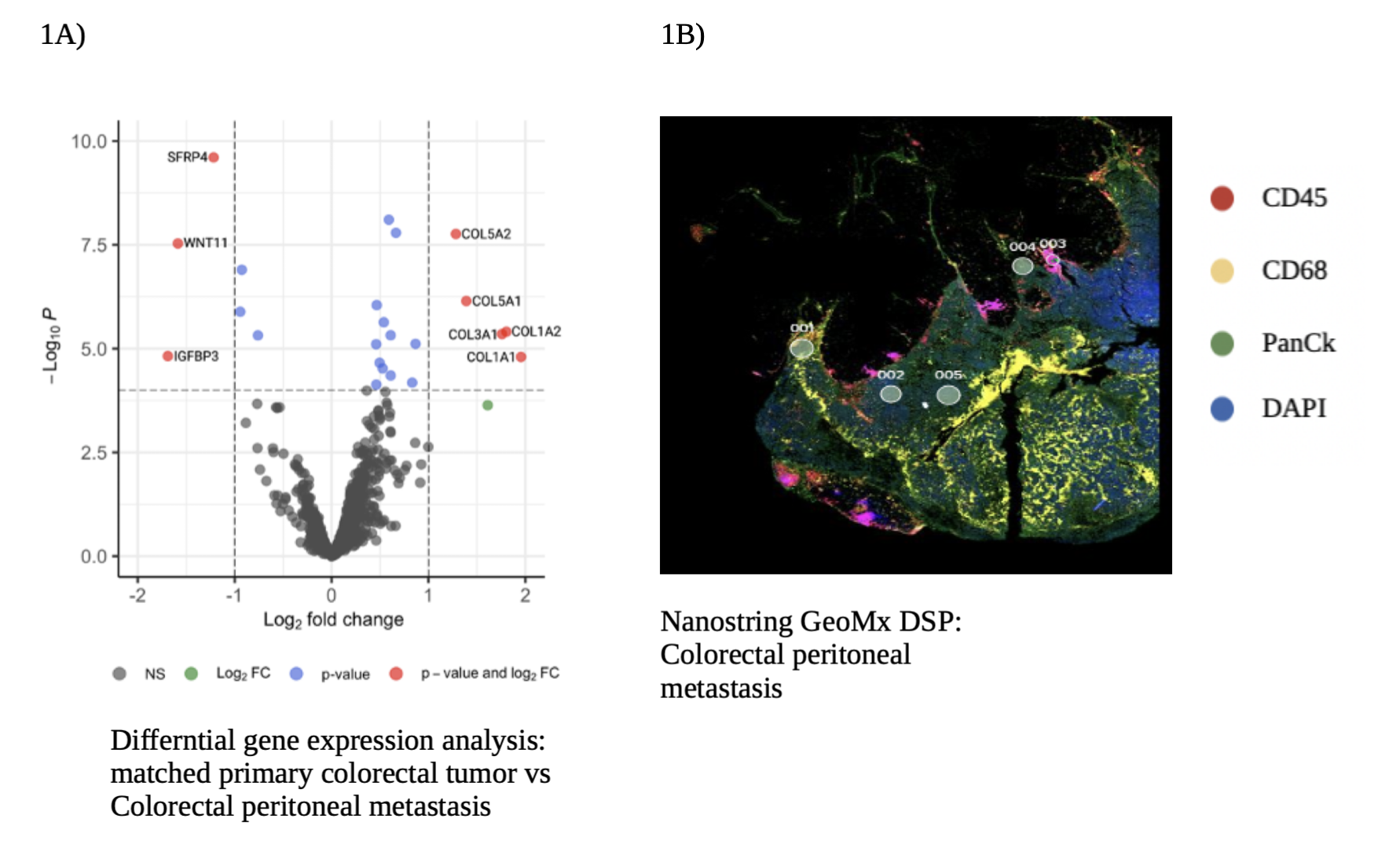
Results: All samples included in the bulk RNA-sequencing analysis were categorized into four groups. Primary tumors (n=21), HM which included liver and lung (n=21), lymph node metastasis (n=10) and CPM (n=18). Collagen gene signature was statistically significant in the CPM when compared to primary tumors and HM (p = 0.008), revealing extensive collagen remodeling in CPM. Transcriptomic analysis on Nanostring GeoMx DSP showed similar results, with a differential expression for COL5A2, COL5A1, COL3A1, COL1A2, COL1A1 genes in CPM when compared to matched primary tumors (Figure 1A). IPA showed an upregulation of extracellular structure organization and collagen fibrils organization pathways in CPM. Spatial visualization of the TME revealed an infiltration of TAMs in CPM, shown as abundant immunofluorescence staining for CD68, a surface protein highly expressed by macrophages (Figure 1B). mRNA expression analysis of CPM showed a preponderance of pro-tumorigenic M2 TAMs, compared to anti-tumorigenic M1 TAMs. Further analysis of M2 TAMs in CPM, showed a significant activation of TGF-β signaling, known to play a key role in extra-cellular remodeling.
Conclusion: CPM are characterized by widespread collagen remodeling and infiltration of tumor associated macrophages. Our study indicates that collagen remodeling in CPM, may be driven by TAMs. More mechanistic studies will be required to validate our results.
P. R. Pannu1, G. S. Yochum2, R. A. Hodin1, W. A. Koltun2, N. Saeidi1 1Massachusetts General Hospital, Surgery, Boston, MA, USA 2Penn State Hershey Medical Center, York, PA, USA
Introduction: Inflammatory bowel disease (IBD), encompassing Crohn’s Disease and Ulcerative Colitis, has been predominantly characterized as affecting Caucasian populations. However, through advancements in research and healthcare access, it is now clear that IBD is increasingly affecting all people, including African Americans. Racial disparities in disease characteristics, severity, and progression have been suggested between African American and Caucasian patients with IBD. However, the potential role of underlying biological factors leading to these racial differences is yet to be unraveled.
Methods: Resected colon samples from 15 African American and 15 Caucasian patients with IBD (16 Crohn’s disease; 14 Ulcerative Colitis) were evaluated. Age-, sex- and duration of disease-matched samples were compared for: 1) Histological damage using Hematoxylin and Eosin (H&E) staining, 2) Extent of fibrosis with Masson’s Trichrome and Collagen staining, and 3) Colon immunophenotyping and cytokine profiling with flow cytometry. Furthermore, we utilized a chronic colitis model (3 cycles of 2.5% DSS for 1 week followed by 2 weeks of water in each cycle) and compared outcomes in pigmented vs non-pigmented C57BL/6J mice.
Results: African American patients showed evidence of higher histological damage when compared with Caucasian patients, seen as more prominent lamina propria immune cell infiltration, crypt architectural distortion and epithelial erosions and ulceration on H&E staining. Additionally, extent of collagen deposition on Masson’s Trichrome (12.5% vs 5.1%, p<0.01) and Collagen I (16.2% vs 2.7%, p<0.01) was significantly higher in African Americans than Caucasians. Furthermore, colon profiling revealed higher levels of pro-inflammatory cytokines like TNF-α and IFN-γ in African American patients compared to Caucasians. Interestingly, pigmented mice developed more severe disease when compared with non-pigmented mice, observed as shorter colon lengths and slower recovery from body weight loss following induction of chronic colitis (p<0.01). Histological analysis of colon tissues from pigmented mice demonstarted more prominent damage characterized by severe disruption of tissue architecture, increased immune cell infiltration and epithelial denudation compared to non-pigmented mice. Similar to human findings, pigmented mice showed higher levels of pro-inflammatory cytokines TNF-α and IFN-γ in colon when compared with non-pigmented mice.
Conclusion: Our findings demonstrate evidence of more severe disease in African Americans with IBD when compared with Caucasian patients. Furthermore, results from the chronic colitis murine model validate its role in investigating the precise underlying mechanisms of IBD, including differences based on pigmentation.
A. B. Littlefield1,2, K. Scheurlen1, A. Macleod1, T. Alfieri1, E. Hinkebein1, S. Galandiuk1 1Price Institute of Surgical Research, Hiram C. Polk Jr. MD Department Of Surgery, University Of Louisville, Louisville, KY, USA 2University of Louisville School of Medicine, Louisville, KY, USA
Introduction: The incidence of colon cancer (CC) in patients younger than 50 years of age has increased by more than 30% in the U.S. since the 1990s. Obesity prevalence has been continuously rising, and obesity contributes to a systemic tumor-promoting proinflammatory state in CC. Peripheral Blood Mononuclear Cells (PBMCs) are inflammatory cells that can be recruited into tumors to form macrophages. In CC, tumor-derived cytokines can affect macrophage differentiation and carcinogenic function to enhance tumor development. PBMCs can alter their gene expression profile while circulating and may function as CC screening markers. Pro- and anti-inflammatory genes play a role in tumor-promoting macrophages and CC progression and can predispose individuals to give rise to carcinogenic macrophages in tumor tissue and therefore CC onset. Inflammatory macrophage markers and transcription factors are associated with prognosis and recurrence rate in patients with CC. The aim of this study was to determine the gene expression profile of inflammatory markers in PBMCs of patients with a diagnosis of sporadic CC compared to healthy controls.
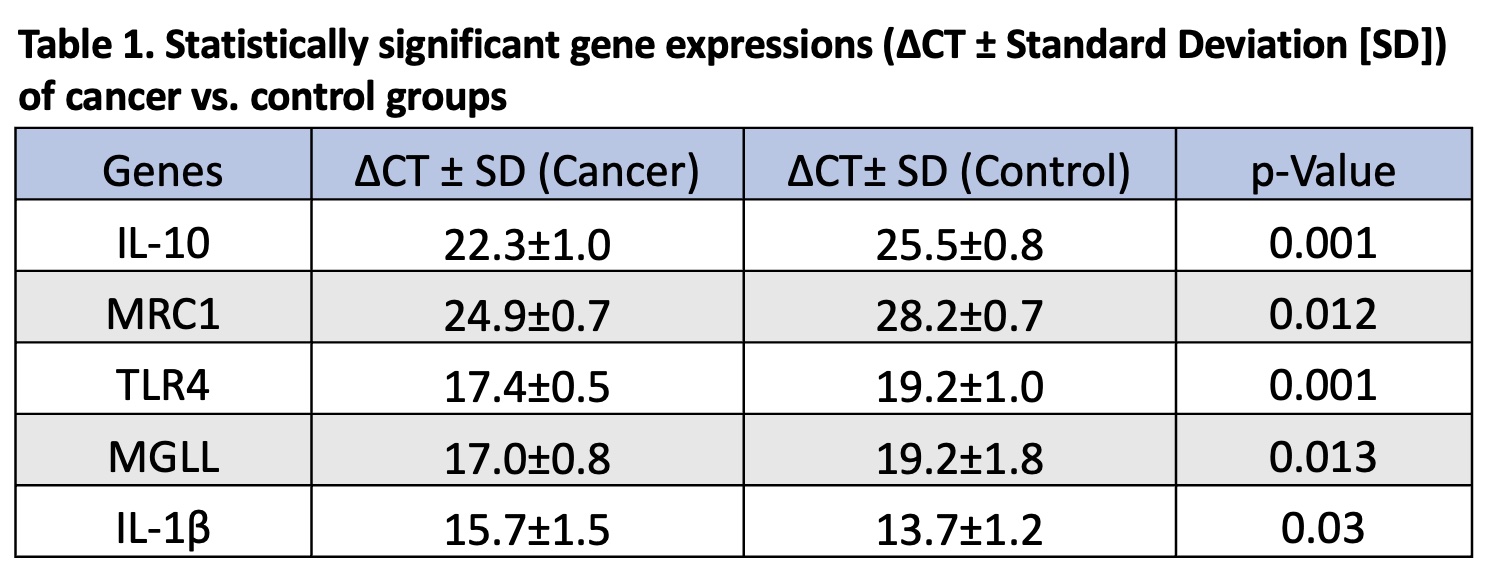
Methods: Blood samples were collected preoperatively from individuals with a diagnosis of sporadic CC undergoing resection (N=8) and from healthy individuals without CC (N=6) via venipuncture using Vacutainer tubes with EDTA. PBMCs were isolated from whole blood using 3% Dextran and Ficoll-Hypaque (1.05 g/ml). TaqMan qRT-PCR was performed to compare inflammatory marker expression. Gene expression is reported using delta CT (ΔCT) values. Values in cancer versus normal samples were compared using the Mann-Whitney U test (p<0.05).
Results: Patient body mass index (BMI) ranged from normal weight (18.5-24.9 kg/m2) to overweight (25-29.9 kg/m2) and one patient had a diagnosis of early-onset CC before the age of 50. Significant expression results are shown in Table 1. The anti-inflammatory and tumor-promoting markers IL-10 and MRC1 were upregulated in the PBMCs of cancer patients compared to healthy controls. The CC-associated marker TLR4 was upregulated in cancer patients. The tumor-suppressing regulator MGLL was upregulated in cancer PBMCs versus controls. Furthermore, tumor-promoting IL-1β was downregulated in cancer PBMCs.
Conclusion: Gene expression of certain tumor-promoting inflammatory cytokines and markers associated with CC progression and survival are elevated in PBMCs of patients with sporadic CC compared to healthy individuals. Expression profiles of genes in PBMC may serve as screening markers in sporadic CC, with a potential role in young and obese patients with early-onset CC.
K. Rouse1, G. Toro2, P. Szaniszlo2, B. Servantes2, M. Hellmich2, K. Modis2 1University Of Texas Medical Branch, School Of Medicine, Galveston, TX, USA 2University Of Texas Medical Branch, Department Of Surgery, Galveston, TX, USA
Introduction: In the United States, colorectal cancer (CRC) is the third most diagnosed cancer and the second leading cause of cancer-related deaths after excluding skin cancer. Almost 50% of patients with CRC will develop metastases, in which prognosis is poor. A critical process in tumor development and metastases is cancer-induced angiogenesis. Anti-angiogenic therapeutics have been developed to treat patients with CRC; however, current therapies have shown varying levels of efficacy due to therapeutic resistance. For this reason, there is an urgent need for novel anti-angiogenic therapies that target different molecules and pathways involved in tumor growth and metastasis. 3-mercaptopyruvate sulfurtransferase (3-MST) is a hydrogen sulfide (H2S)-producing enzyme, that is abundantly expressed in endothelial cells (ECs). Significantly elevated levels of H2S have been demonstrated in CRC, however, the role of the 3-MST/H2S axis in tumor angiogenesis remains unknown. This study aims to characterize the regulatory role of EC 3-MST in promoting CRC-associated angiogenesis, as a potential novel anti-angiogenic therapeutic target.
Methods: To assess in vitro angiogenic responses, EA.hy926 human vascular ECs subjected to shRNA-mediated 3-MST were used. Proliferation, migration, and tube-like network formation abilities of sh3-MST ECs were tested by subjecting ECs to complete HCT116 growth media and HCT116 cell-conditioned media (1.7 million cells cultured in 30 mL for 120 hrs). Quantification was performed by measuring the number of tubes, tube length, and determining the tube area (ImageJ 1.45s software, National Institutes of Health). The functional role of EC 3-MST in colon tumor growth, angiogenesis, and metastasis was defined in vivo by 3-MST knockout (KO) mice injected with MC38 murine colon adenocarcinoma cells that were assessed at 6-, 9-, and 12-days post-injection versus wild-type mice.
Results: 3-MST attenuation in ECs significantly reduced EC proliferation, migration, and tube-like network formation in vitro. In addition, sh3-MST ECs developed significantly fewer tubes in HCT116 cell-derived conditioned media. Immunocompetent mice subcutaneously injected with MC38 murine colon adenocarcinoma cells showed increased tumor take in wild-type mice at earlier time points post-injection than 3-MST KO mice.
Conclusion: Reduced 3-MST activity in ECs alters angiogenic mechanisms. The data presented in this study supports the view that the 3-MST/H2S signaling pathway could serve as a potential candidate for future cancer therapies.
L. C. Gunder1, H. A. Green2, A. Bilger3, H. R. Johnson1, T. H. Moyer1, E. H. Carchman1,2,4 1University Of Wisconsin, Department Of Surgery, School Of Medicine And Public Health, Madison, WI, USA 2University Of Wisconsin, Carbone Cancer Center, School Of Medicine And Public Health, Madison, WI, USA 3University Of Wisconsin, McArdle Laboratory For Cancer Research, Department Of Oncology, Madison, WI, USA 4William S. Middleton Memorial Veterans Hospital, Madison, WISCONSIN, USA
Introduction: Infection of the mouse anal tract with mouse papillomavirus (MmuPV1) is an innovative preclinical model that allows for the study of viral-mediated anal disease. Immunodeficient mice infected with papillomavirus, similar to immunosuppressed human patients, have difficulties in successfully clearing the virus. The goal of this project is to assess the effect of the topical protease inhibitor, Saquinavir, on MmuPV1 viral RNA in a mouse model of viral-mediated anal disease and its relation to tissue concentrations of the drug.
Methods: Male and female immunodeficient NOD scid gamma (NSG) mice (8 -16 weeks of age), were infected in the anus with 3×108 viral genome equivalents of MmuPV1. After 20 weeks, mice received the following topical treatments to the anal canal: 1% solution of Saquinavir (SQV) five days a week and/or the carcinogen, 0.12 μmol 7,12 dimethylbenz(a)anthracene (DMBA) weekly. After 20 weeks of treatment, anal tissue was harvested for quanitifcation of SQV by electrospray ionization mass spectroscopy. A portion of anus underwent RNA in situ hybridization (RNA-ISH) targeted to MmuPV1. SQV quantification between groups was compared by unpaired t-tests. One-way ANOVA was utilized to analyze RNA levels (measured in optical density) with Tukey’s multiple comparison tests between groups. Correlation between viral RNA and SQV levels was assessed via Pearson’s correlation coefficient.
Results: Tissue from mice treated with SQV and DMBA (n = 15) had significantly higher levels of SQV (190.1 mg/g) present than the SQV alone (85.75 mg/g; n = 15; P = 0.0144). This effect only remained statistically significant in female mice tissue (266.4 v. 96.34 mg/g; Female P = 0.0139; 123.2 v. 73.64 mg/g; Male P = 0.7711). Male tissue had significantly lower levels of SQV (P = 0.0460) when comparing tissue from males treated with SQV and DMBA to females with SQV and DMBA.
There were no significant differences in viral RNA between treated with SQV compared to respective controls with or without DMBA and regardless of sex (mean viral RNA value range for all treatment groups = 0.3014 – 0.3801 optical density; P > 0.05). There was no correlation between viral RNA and SQV levels between groups when looking at both males and females together (P > 0.05). However, in females treated with SQV alone, there was an inverse correlation between levels of SQV and viral RNA (r = -0.8596; P = 0.0282).
Conclusions: Treatment with topical SQV had a greater effect in MmuPV1-infected female mice than male mice in terms of decreased tissue viral RNA. This may be potentially related to the fact that tissue from female mice had significantly higher levels of SQV present compared to males. Increased SQV in the tissue of female mice treated with SQV only correlated with reduced viral RNA.
A. Hu1, J. Mitchem2, Y. Nussbaum2, J. Yoo1 1Brigham And Women’s Hospital, General And GI / Surgery, Boston, MA, USA 2University Of Missouri, Surgery, Columbia, MO, USA
Introduction: Dynamic cell-cell interactions shape the tumor microenvironment to regulate tumor growth and invasiveness. Myofibroblasts are gastrointestinal stromal cells that are upregulated in the setting of colorectal cancer (CRC) and may play an important role in tumor-stromal cell communication. Angiogenin is a 14-kDa ribonuclease that regulates myofibroblast function and has been implicated in myofibroblast-CRC cell communication in mouse models. However, its role in human patients has not been well established.
Methods: Open access, annotated single-cell RNA sequencing data of paired normal human colon and colorectal cancer tissue (n=20) was available in the NCBI Gene Expression Omnibus Database. CellChat was utilized to quantitatively infer biologically meaningful cell-cell communication networks from scRNA-seq data. PLXNB2 and ACTA2 are cell surface angiogenin receptors that regulate angiogenin signaling. Ligand-receptor interactions involving angiogenin, PLXNB2 and ACTA2 were analyzed between cell populations in each sample.
Results: We found no difference in overall angiogenin expression comparing normal colon and CRC tissue. However, notable differences were identified in the types of cells that express angiogenin, with a shift in angiogenin expression from the stroma under normal conditions to the epithelium in the presence of cancer. In normal colon, myofibroblasts do not express angiogenin or the PLXNB2 receptor. Despite an overall decrease in angiogenin expression in the stroma, CRC-associated myofibroblasts were characterized by a significant upregulation of both angiogenin and PLXNB2 receptor expression (P<0.05), while no difference was seen in ACTA2. CRC cells not only utilize angiogenin for autocrine signaling but also to communicate with myofibroblasts via the PLXNB2 receptor (P<0.05) and ACTA2 (P=0.0647).
Conclusion: Compared to normal human colon tissue, CRC is associated with an enrichment of myofibroblasts that exhibit upregulated expression of angiogenin and the angiogenin receptor PLXNB2. CRC cells engage in autocrine signaling via angiogenin, as well as paracrine signaling with myofibroblasts via PLXNB2. Angiogenin is directly involved in tumor-stromal cell communication in human colon tissue and may play an important role in colorectal cancer progression.
H. Johnson1, L. Gunder1, T. Moyer2, M. Ziolkowski1, P. Bertrang1, A. Bilger3, E. Carchman1 1University Of Wisconsin, Department Of Surgery, Madison, WI, USA 2University Of Wisconsin, Waisman Center, Madison, WI, USA 3University Of Wisconsin, School Of Medicine And Public Health, Madison, WI, USA
Introduction: The development of anal dysplasia is associated with infection of anal tissue with human papillomavirus (HPV). Anal dysplasia has the potential to progress to anal cancer without treatment. We have found that the topical application of the protease inhibitor, Saquinavir, prevents the progression of anal dysplasia to anal cancer in an immunocompetent transgenic mouse model. We hypothesized that topical Saquinavir would promote viral clearance in an immunocompromised NOD scid gamma (NSG) mouse model infected with Mus musculus papillomavirus (MmuPV1). This novel infectious mouse model allows us to study viral clearance.
Methods: Male and female NSG mice were infected in the anus with 10^8 viral genome equivalents of MmuPV1 and began treatment 140 days post-infection to allow time for progression to high-grade anal dysplasia. Mice were randomized into four treatment groups, which included: control (mock, infected with MmuPV1, but left untreated), 7,12 dimethylbenz(a)anthracene only (DMBA (0.12 μmol)), Saquinavir only (SQV (1%)) (dissolved in dimethyl sulfoxide), and Saquinavir with DMBA (SQV + DMBA). Mice were treated topically at the anus with Saquinavir, 5 days per week and DMBA once weekly, for 20 weeks. Prior to initial treatment and again immediately before sacrifice, mice anal tracts were lavaged with phosphate buffered saline and DNA was extracted from the lavages. MmuPV1 viral load was measured using qPCR. Viral load data was analyzed with one-way ANOVA and paired t-tests.
Results: All of the treatment groups showed no significant difference in initial viral loads prior to receiving treatment regardless of sex. There were no differences in female or male control mice when comparing initial to final viral load. However, female mice had a statistically significant decrease in mean viral load following treatment (initial versus final) in both the SQV (P = 0.0198) and SQV + DMBA groups (P = 0.0033). Male mice had an increase in mean viral load from initial to final lavage in the SQV + DMBA (P = 0.0219) treatment group. There was no difference in mean initial and final viral load found in male mice in the SQV alone group.
Conclusion: Female NSG mice infected with MmuPV1 and treated with topical Saquinavir had a significant decrease in mean viral load in final lavage compared to initial lavage. We suspect the increase in viral load seen in male mice following SQV+ DMBA may be related to the ability of male mice to re-infect cage mates via anal penetration and/or epithelial damage from DMBA or dimethyl sulfoxide (the diluent). Further experiments with singly housed male mice are needed to confirm this hypothesis and exclude sex differences. These results could have implications for determining treatment length and regimen when considering the possibility of viral re-infection in humans.
J. B. George1, A. Macleod1, K. Scheurlen1, D. Snook1, C. Barbour1, S. Galandiuk1 1University Of Louisville, Price Institute Of Surgical Research, Hiram C. Polk Jr. MD Department Of Surgery, Louisville, KY, USA
Introduction: Colon cancer is the third most prevalent cancer and the second leading cause of cancer related death in both men and women in the United States. In late-stage colon cancer, the 5-year survival rate is 10% due to the limited response to standard treatments. Immunotherapy is an effective treatment option for many cancers such as melanoma, renal and lung cancer. Anti-PDL1 therapy is a type of immunotherapy that targets the PDL1/PD1immune proteins on the cell surface. In colorectal cancer, anti PDL1/PD1 therapy has been a promising treatment in mis-match repair deficient (MMRd) cancers. However, in mis-match repair proficient (MMRp) cancers, which accounts for 85% of colorectal cancers, its success has been poor. MMRp cancers have low PDL1 expression. PDL1 can be upregulated in cells by Interferon Gamma (IFNγ) treatment. The aim of this study is to characterize PDL1 expression in colon cancer cell line SW480 (MMRp) and measure its response to IFNγ treatment.
Methods: SW-480 (Stage II) colon adenocarcinoma cells were plated in 24-well plates. Cells were treated with 0, 10, 100 or 500 ng/ml of IFNγ and harvested after 0, 3, 6, 18, 24, 48 hours (n=5 for each dose and time point). Following cell and supernatant harvest, qRT-PCR was performed on mRNA. PDL1 ELISA was used to measure cell lysate and supernatant protein levels. Following staining with anti-PDL1 antibodies flow cytometry analysis was performed with a FACS Calibur flow cytometer. Data were analyzed using the Kruskal-Wallis Test (p<0.05).
Results: IFNγ upregulated PDL1 gene expression at all times and doses. The optimal time for increased gene expression was 6 hours for both 100ng/ml (9.7±1.7-fold-change, p=0.032) and 500ng/ml (14.1 ± 1.9 fold-change, p=0.026). Cell surface protein expression was also increased following treatment. At baseline 18.4 ± 4.9 % of SW480 cells expressed PDL1. This increased to 89.8 ± 2.94% following treatment with 500ng/ml IFNγ and was maintained for 48 hours (p=0.02). No difference was seen in PD-L1 protein expression in cells treated with 100 vs 500ng/ml (p=0.592). Extracellular PDL1 expression was unaffected by IFNγ.
Conclusion: SW-480 colon cancer cells express PDL1. IFNγ treatment upregulates PDL1 gene expression, as well as PDL1 cell surface protein levels. Increased cell surface expression is maintained for at least 48 hours. This provides an important baseline upon which further cell culture studies can be based to examine the effect of cancer cell PDL1 expression on other cell types within the tumor micro-environment. In the future we will compare SW-480 (MMRp) PDL1 expression and upregulation to that of the (MMRd) cell line, Hct-116.
A. M. Beierle1, H. R. Markert1, C. H. Quinn1, J. R. Julson1, J. E. Stewart1, N. F. Reuel2, E. A. Beierle1 1University Of Alabama at Birmingham, Pediatric Surgery, Birmingham, Alabama, USA 2Iowa State University, Chemical And Biological Engineering, Ames, IA, USA
Introduction: Inductor-capacitor resonant sensors have been applied to numerous biological conditions in rodent models including monitoring wound healing, characterizing viable and non-viable abdominal tissues, detecting abdominal tumor metastases, and differentiating various forms of pediatric solid tumor xenografts. We aimed to determine whether the resonant sensor system could be applied to human infants to detect changes in the gastrointestinal tract in a non-invasive fashion. We hypothesized that there would be a significant difference in the resonant frequency between normal and abnormal human intestinal tissue.
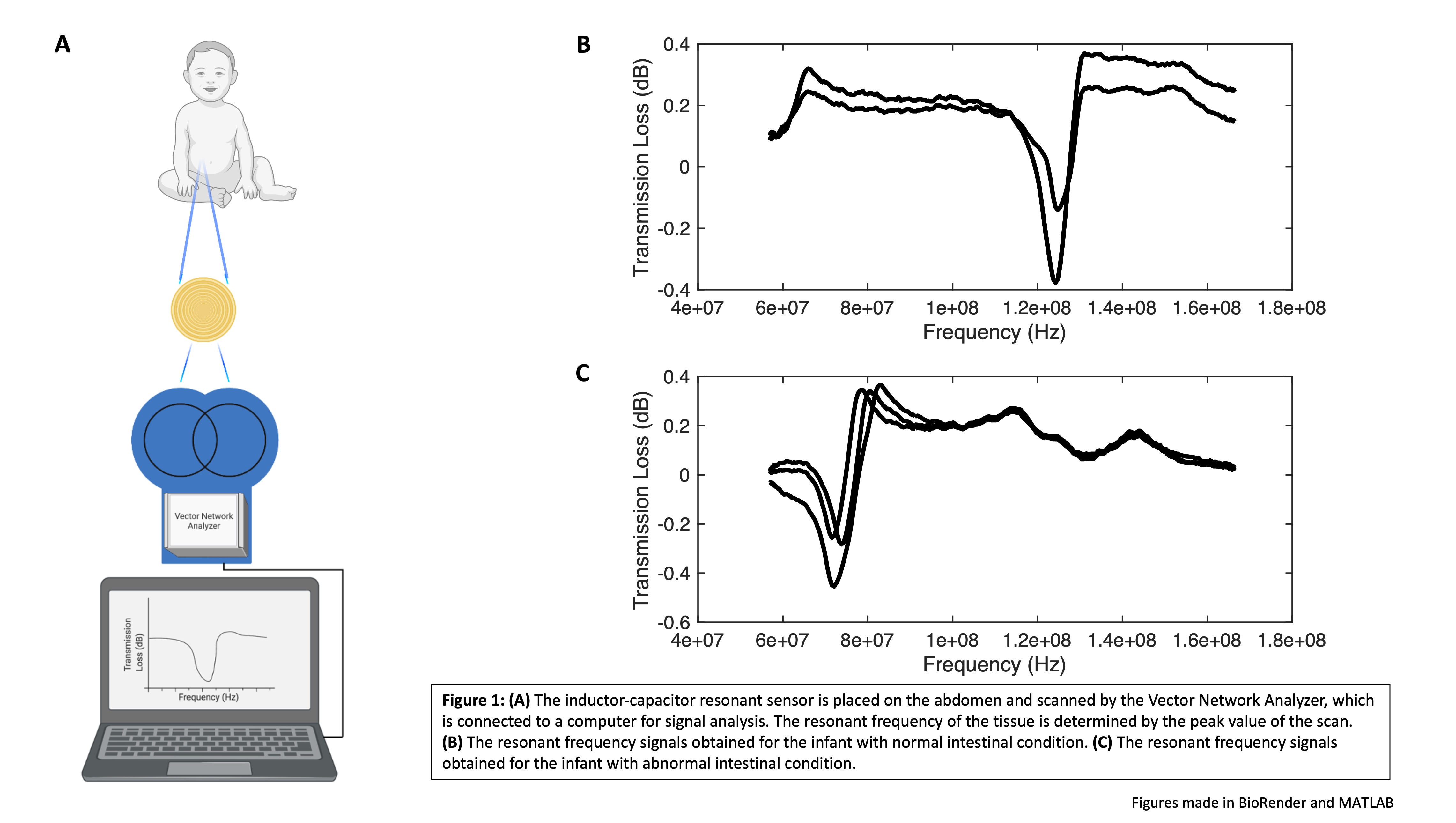
Methods: The resonant sensor system consisted of an inductor-capacitor resonant sensor embedded in Tegaderm film. The resonant sensor was scanned using a Vector Network Analyzer which allowed for the determination of the resonant frequency of the tissue in question (Fig 1A). Following institutional IRB approval, the resonant sensor was placed on the lower abdomen of infants under post-operative care to determine the resonant frequency value of intestinal tissue (n = 10). The resonant frequency of the tissue was determined three times for each location. Data points from two individual infants were further analyzed. These data compared the resonant frequency values from a 17-week-old infant being treated for feeding difficulties secondary to a cleft palate (normal intestinal tissue), to those of a 5-week-old infant who was treated for gastroschisis (abnormal intestinal tissue). The data were analyzed using MATLAB Mathworks Software and significance determined at p < 0.05.
Results: The inductor-capacitor resonant sensor demonstrated the capacity to characterize differences between resonant frequency values of normal and abnormal infant intestinal tissue. The resonant frequency obtained for the baby with normal intestinal tissue was 1.2 x 108 ± 7.5 x 105 Hz (Fig 1B). The resonant frequency obtained for the baby with abnormal intestinal tissue was 7.5 x 107 ± 6.7 x 105 Hz (Fig 1C). The p value for this comparison was p < 0.0001, indicating a significant difference between the resonant frequency values obtained.
Conclusion: Utilizing an inductor-capacitor resonant sensor applied to the lower abdomen of infants, we were able to determine the resonant frequency value of intestinal tissue. The resonant sensor system detected a significant difference between the value of normal and abnormal intestinal tissue. These preclinical human data indicate that resonant sensor technology should be further investigated for its potential clinical applications for real-time non-invasive monitoring of various gastrointestinal conditions in infants and children.
J. Peiro1, M. Miyabe1, S. Duru1, J. Hawes2, C. Lin2, M. Oria1 1Cincinnati Children’s Hospital Medical Center, Pediatric Surgery, Cincinnati, OH, USA 2University Of Cincinnati, Orthopaedic Surgery, Cincinnati, OH, USA
Introduction: The objective of this study was to compare the surgical functionality for duraplasty between our new designed PLA/PCL “smart patch” and another one, as the cryopreserved human umbilical-cord (HUC) matrix, in the fetoscopic myelomeningocele repair by using a fetoscopic surgical simulator.
Methods: A fetoscopic box trainer with inanimate silicone fetus doll, previously used in our laboratory for surgical simulation for training our learning curve and compare 2D and 3D scopes (Patel, 2020), was used to compare the performance of PLA/PCL vs HUC patch during prenatal myelomeningocele repair. The silicon doll was wrapped with chicken skin to simulate a lumbar defect. Participants with varying surgical experience were divided in three groups considering their laparoscopic knowledge (novice, intermediate and expert) and were asked to complete four tasks: patch introduction thru-trocar, manipulation placement of the patch subcutaneously, and skin closure. All completed the four tasks using both patches randomized in different days. Time to completion was measured, and the participants subsequently completed the NASA Load Index test and questionnaires evaluating their experiences.
Results: Twenty participants completed the tasks twice. Eight were classified as novice (40%), 4 as intermediate (20%) and 8 as experts (40%). The “patch introduction” task was faster with PLA/PCL patch in all groups but with statistical significance in the “novice” group with 16.88 seconds for PLA/PCL vs 39.50 seconds for the amniotic membrane (p=0.010) and the ‘experts” group with 11.4 seconds for the PLA/PCL vs 23.9 seconds for the amniotic membrane group (p=0.019). For the other three tasks there was no statistically significant differences. 19/20 participants (95%) referred subjective preference for the PLA/PCL patch.
Conclusion: Smart PLA/PCL patch with its preformed coiled tube shape and self-expandable property by body-temperature activation facilitates its introduction thru the trocar compared to the HUC patch. The similar functionality in the other tasks shows that the smart PLA/PCL patch is a great candidate as dural substitute in fetoscopic myelomeningocele repair.